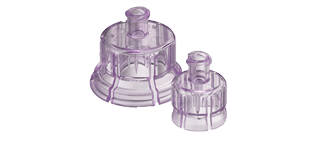
West’s Vial Adapters – A Safer Solution for Handling Cell Therapy Applications
Administration of cell therapy drug products requires the delivery of cell suspensions without loss of function or viability. Cells must be transferred aseptically from their original container closure system to the administration system by infusion. However, the common transfer practice of using needles and syringes to pierce vial stoppers to retrieve contents poses a risk, namely needle-stick injury to clinicians.
![]()

West has a solution – polycarbonate-based, needle-free, vial adapters (VAs) that are compatible with vials made of either glass or Daikyo Crystal Zenith® cyclic olefin polymer (a high-quality engineered polymer). VAs enable aseptic transfer from the vial through a Luer-lock connection. Vented VAs allow sterile filtration of air to replace liquid as it is withdrawn, avoiding pressure differentials. Use of VAs is convenient and reduces risk of needle-stick injuries. Moreover, VAs provide for cell function and viability. This was the subject of a recent report by West scientists: Evaluation of West’s Vial Adapters for Cell Therapy Applications, Technical Report 2020/224. It was shown that polycarbonate is compatible with DMSO-based cell media. And in collaboration with scientists at Loughborough University (a major national center in the UK for innovative and process-oriented regenerative medicinal research), retention of both function and viability was demonstrated with two types of stem cells, mesenchymal and hematopoietic. After freeze/thaw processing, cell recovery and cell viability were excellent and provided equivalent results whether stem cells were withdrawn by VAs or the standard method using needles. Performance was independent of operator – indicating ease of VA use.
VAs were demonstrated to be a suitable option for cell therapy drug products and were reported to be preferred in handling over the commonly used needles. They are an important aspect of West’s overall support for cell therapy drug products. For more on how West can help visit our Vial Adapters webpage or contact an Account Manager or Technical Customer Support representative.
Disclaimer:
Vial Adapters are 510(k) cleared by the United States Food and Drug Administration and carry the CE mark (0344). Products are shown for INFORMATION purposes only and may not be approved for marketing in specific regions. Distribution and use are subject to applicable regulatory approvals and requirements for medical devices. Please contact your West Pharmaceutical Services, Inc. (West) representative for product availability. Important product information, safety and warnings are included at: https://www.westpharma.com/products/vial-adapter-systems/vial-adapters.
Crystal Zenith® is a registered trademark of Daikyo Seiko, Ltd.
Daikyo Crystal Zenith® technology is licensed from Daikyo Seiko, Ltd.