West Recognizes World Cancer Day 2021
Cancer affects all of us, whether we are a patient, a family member, a friend, or a colleague of someone fighting cancer. Working together, we can increase our understanding of this disease and support the groundbreaking research that is ongoing in the fight against cancer.
![]()

February 4 is World Cancer Day, a global initiative of the Union for International Cancer Control (UICC). This year’s World Cancer Day theme is “I Am and I Will,” which underscores the importance of everyone taking actions to reduce premature deaths from cancer. These combined efforts are designed to move our communities closer to a healthier, brighter world without cancer. Click here to learn ways you can take action and raise awareness.
West is incredibly proud to partner with customers to Simplify the Journey™ of oncology drug development, with a shared goal of helping patients receive life-saving treatments. Pharmaceutical pipelines are highly focused on oncology therapies, and these molecules need to be brought to market in varying packaging and delivery formats based on the needs of the molecule, the patients, and the delivery requirements. More than 70 oncology products are expected to launch in the next five years, all with unique needs!
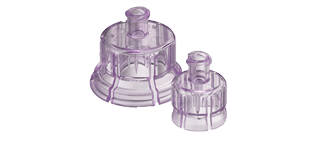
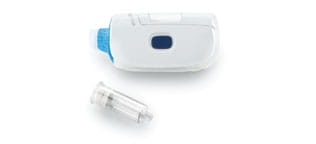
West has supported the journey of many oncology drugs, including Amgen’s IMLYGIC™ (talimogene laherparepvec) for the treatment of melanoma, which utilizes West’s high-quality vial packaging system. Many cancer medications are already approved for use with West’s needle-free reconstitution systems, such as the Vial Adapter and the MixJect® device. Our Vial Adapters guide spike insertion, which can reduce coring and fragmentation associated with multi-dose rubber stoppers. They also reduce risk of needlestick injuries and enable multiple vial accesses*. The MixJect device enables easy reconstitution, vial-to-PFS transfer, and injection of medicines. Upon reconstitution, the drug is available for immediate injection with a pre-attached needle.
We are all working to make an impact in the search for effective cancer treatments, so it’s vital that we can provide valuable solutions to our oncology customers, taking into account the specific needs of their drugs. Our team members are very proud to work for a company that helps its customers make an impact in the lives of cancer patients every day.
Please click here to learn more about how West helps customers navigate the challenges of oncology drug development.
*Swabable vial adapter variations only.
Vial Adapters(TM) are 510(k) cleared by the United States Food and Drug Administration and carry the CE mark (0344). Products are shown for INFORMATION purposes only and may not be approved for marketing in specific regions. Distribution and use are subject to applicable regulatory approvals and requirements for medical devices. The Vial Adapter(TM) is configurable and may not be suitable for use with all drugs. Refer to drug manufacturer's labeling and use instructions for device configuration compatibility. Failure to follow product instructions for use may result in compromised sterility; contamination; leakage (including possible exposure to medication); and/or inadequate medication reconstitution, transfer, and/or dosing. Product misuse could potentially lead to user and/or patient infection, suboptimal therapy, and/or delay in therapy. Please contact your West Pharmaceutical Services, inc. (West) representative for product availability. Important product and safety information and warnings here: https://www.westpharma.com/-/media/westpharma/files/products/vial-adapter-indications-safety-and-warnings.pdf
The MixJect® device is 510(k) cleared by the United States Food and Drug Administration and carries the CE mark (0344). Products are shown for INFORMATION purposes only and may not be approved for marketing in specific regions. Distribution and use are subject to applicable regulatory approvals and requirements for medical devices. The MixJect® transfer device is configurable and may not be suitable for use with all drugs. Refer to drug manufacturer's labeling and use instructions for device configuration compatibility. The device may contain a pre-attached needle containing cobalt. Injection site pain, irritation, discomfort, bruising, and/or erythema may occur. Failure to follow product instructions for use may result in compromised sterility; contamination; leakage (including possible exposure to medication); and/or inadequate medication reconstitution, transfer, and/or dosing. Product misuse could potentially lead to needlestick injury, user and/or patient exposure to pathogens or infection, and/or suboptimal or delayed therapy. Please contact your West Pharmaceutical Services, inc. (West) representative for product availability. Important product and safety information and warnings at: https://www.westpharma.com/-/media/westpharma/files/products/mixject-indication-safety-and-warnings.pdf
Simplify the Journey™ is a trademark of West Pharmaceutical Services, Inc. in the United States and other jurisdictions.
MixJect® is a registered trademark of West Pharma. Services IL, Ltd., a subsidiary of West Pharmaceutical Services, Inc.