Combination Products: The Top Three Device Challenges
Combination products open up new vistas when it comes to delivering innovative therapies to patients in need, but teams bringing these products to the market face a number of significant hurdles. Recent surveys conducted by West at workshops held in PA and AZ have revealed some of our customers’ biggest challenges. This is the second of three blogs focused on these challenges — and how West can help Simplify the Journey™ when it comes to developing a combination product.
![]()
The demand for combination products is growing because they make the patient’s life easier, but they can present a number of challenges during development. West stands by the side of our customers to help them understand and navigate these challenges.
To that end, West recently held customer workshops in Pennsylvania and Arizona, focused on defining strategies for combination products. We heard attendees identify their biggest challenges, which fell into three categories: regulatory, device and testing/reporting. In this blog, the second in a series, we focus on some of the device-related challenges that resurface for customers again and again. Addressing these three challenges can help ensure patient safety and device success.
Device Challenges
For combination products, device-related issues can be far-ranging, but in our survey results, we discovered some common themes:
1. Device Reliability and Robustness
A pharmaceutical or biologic can’t help a patient if it isn’t delivered safely, accurately and consistently. A robust, reliable device is the vehicle for this delivery, and the design control process is the pathway to ensure that the device does its job properly. A critical step is verification testing, which confirms that the device has been designed correctly and that it performs reliably against defined patient requirements and drug specifications. It also evaluates the integrity of the product in worst-case conditions that could include drops, temperature extremes and exposure to moisture.
Human factors testing — how end users interact with a device — is also important to ensure device reliability. FDA guidance focuses on three aspects: the intended users, the use environments, and the user interface. As a result, regulators look for evidence of safe and effective use of your product from these three perspectives.
2. Drug and Device Integration
The way the device impacts the drug and vice versa are important considerations when developing combination products. The FDA lists nine different types of combination products, which range from a prefilled drug device or delivery system to a device coated, impregnated, or combined with a drug or a biologic. The precise combination of elements, as well as the critical interfaces, can shape the development process significantly.
Drug and device development teams must work seamlessly together, and educate each other, on the requirements of each element to bring a successful product to market. For example, companies must understand the mechanics of drug dispersion — will the drug be administered subcutaneously, via transdermal patch, or by aerosol? — to figure out how the device could impact drug delivery. Material selection can also impact drug delivery and performance. These critical issues should be identified, quantified, and stabilized early in the combination product’s development.
3. Accommodating Different Volumes
Patient self-administration of large drug volumes using traditional devices, such as autoinjectors, pens or prefilled syringes, has been challenging, but the invention of wearable devices is changing the game, allowing larger volumes to be administered subcutaneously over a longer period. This expands possibilities for patient-centered health but creates a number of manufacturing and packaging challenges. Drug development teams must be able to accommodate the evolving demands of higher-volume, higher-viscosity systems, which can impact a number of variables, from device engineering and the device-formulation interface to a variety of human factor issues, including use scenarios and use-related risks.
Simplify the Journey™
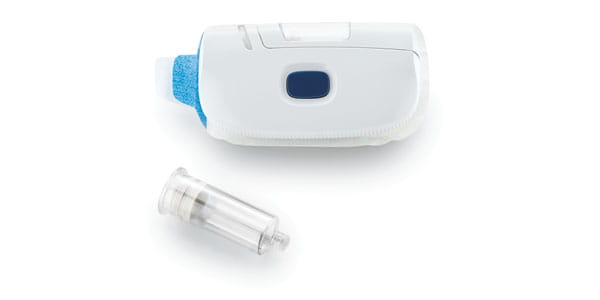
Drug manufacturers have a long and complex path when bringing a combination device to market. West can help through our Integrated Solutions program, offering expertise on contract manufacturing, regulatory, device, analytical, and packaging issues. Our experience can be seen in groundbreaking combination products, such as the SmartDose® drug delivery platform, which has evolved through three generations to accommodate changing patient and drug delivery needs. Count on West to help you Simplify the Journey™ at any stage of drug development.
Please join us for the final installment in this series of blogs: The Top Three Testing/Reporting Challenges for Combination Products.
Simplify the Journey™ is a trademark of West Pharmaceutical Services, Inc., in the United States and other jurisdictions.
SmartDose® is a registered trademark of West Pharma. Services IL, Ltd., a subsidiary of West Pharmaceutical Services, Inc.,