Performance of NovaPure® Plungers for Syringes in Autoinjector Systems
An important element of drug product lifecycle management is anticipating use of delivery systems. When moving from vial systems to pre-filled syringes and autoinjector systems, factors to consider include patient and caregiver needs, drug product requirements, system components, and performance. This article presents performance of West’s 1-3mL and 1mL Long NovaPure® plungers versus legacy plungers for pre-filled syringes in autoinjectors; both plungers are formulation 4023/50 Gray. Performance of the NovaPure® plungers was equivalent to or better than legacy plunger performance in all aspects. Coupled with the enhanced features of NovaPure® plungers, this work indicates they are suitable when best performance is needed.

Introduction
As drug products move through their lifecycle, more manufacturers are adopting pre-filled syringes and self-administration systems, such as autoinjectors, due to their ease of use and functionality. Benefits of self-administration systems include fewer steps in usage as drug product does not need to be withdrawn from a vial, lower costs from fewer/eliminated visits to hospital/clinic, more convenience for patient lifestyle, better safety with automatic needle retraction, and better compliance through connectivity such as smartphone apps.
Pre-filled syringes are critical components of self-administration systems. Use of pre-filled syringes affords better delivery accuracy and more efficient drug product use over vial systems (vials cannot be emptied completely). A key element of a pre-filled syringe is the plunger component. West’s 1-3mL and 1mL Long NovaPure® plungers were designed according to quality by design (QbD) principles for use in pre-filled syringes – especially those coupled with autoinjectors. They are produced with enhanced process and dimensional controls, vision inspection on each component, FluroTec® film contacting drug product, and defined low particulate levels.
Performance, including accuracy of delivered volume, injection (delivery) time, break loose and glide force (BLG) performance, and work of extrusion, of the 1-3mL and 1mL Long NovaPure® plungers has been examined versus their respective legacy plunger designs over 12 months with placebos of different viscosities (1.1, 8, 15 cP) representative of common biologic drug formulations, in a laboratory configuration that replicates an autoinjector.
All plungers comprised formulation 4023/50 Gray. For performance testing, the 1-3mL NovaPure® and legacy ART 2345 plungers, were assembled in 2.25 mL staked needle siliconized glass syringes and filled to a nominal volume of 2.25 mL with different viscosity solutions (1.1, 8, 15 cP). The 1mL Long NovaPure® and legacy ART 2340 plungers were assembled in 1 mL long staked needle siliconized glass syringes and filled to a nominal volume of 1.0 mL with the same viscosity solutions. Measurement of delivered volume accuracy and injection time employed a laboratory autoinjector; measurement of BLG performance employed an Instron instrument.
Results
Results of delivered volume are shown in Figures 1-2.
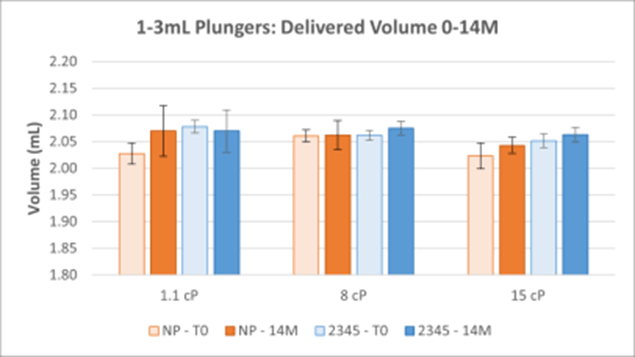
Figure 1. Delivered Volume Results for 1-3mL NovaPure® (NP) and Legacy ART 2345 Plungers at Three Viscosities. The average of 30 samples with standard deviation are shown for each timepoint. 12M samples were delayed to 14M due to equipment issues.
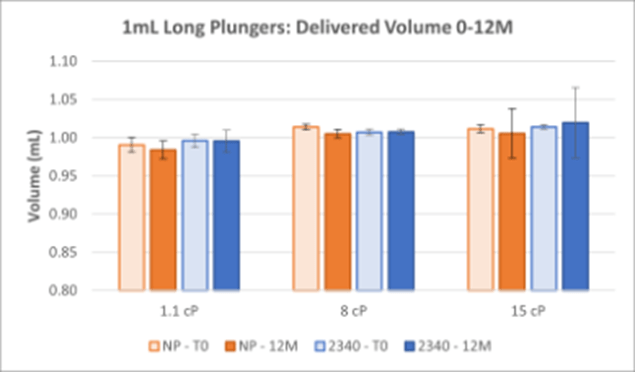
Figure 2. Delivered Volume Results for 1mL Long NovaPure® and Legacy ART 2340 Plungers at Three Viscosities. The average of 30 samples with standard deviation are shown for each timepoint.
Delivered Volume:
- Delivered volume results show that 1-3mL and 1mL Long NovaPure® plungers have equivalent accuracy (in terms of volume delivered) and precision (comparable standard deviation) as compared to ART 2345 and ART 2340 plungers, respectively. There was no variation based on viscosity.
Results of injection time (also known as delivery time) measurements are shown in Figures 3 - 4.
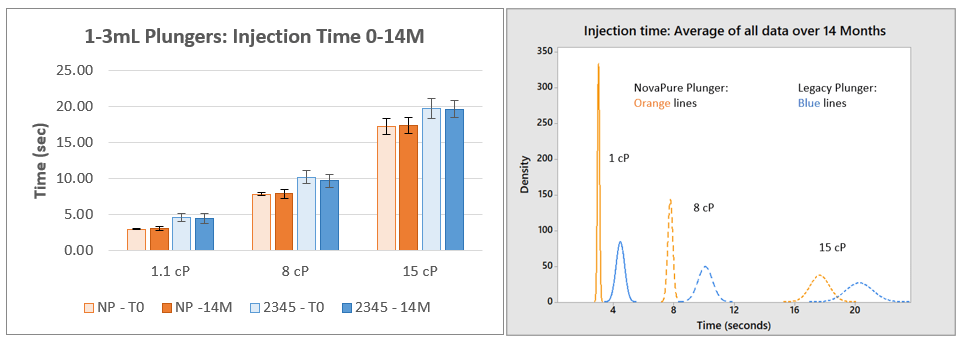
Figure 3. Injection Time Results for 1-3mL NovaPure® (NP) and Legacy ART 2345 Plungers at Three Viscosities. The average of 30 samples with standard deviation are shown for each timepoint and corresponding histogram. 12M samples were delayed to 14M due to equipment issues.
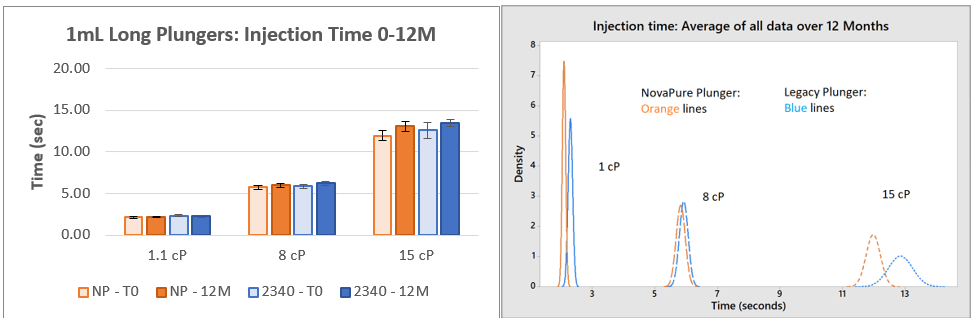
Figure 4. Injection Time Results for 1mL Long NovaPure® and Legacy ART 2340 Plungers at Three Viscosities. The average of 30 samples with standard deviation are shown for each timepoint and corresponding histogram.
Injection Time:
- Injection time data indicate that 1-3mL NovaPure® plungers have consistently faster injection times and less variation (demonstrated by lower standard deviation) than ART 2345 plungers for all viscosities. 1mL Long NovaPure® plungers have equivalent injection times and standard deviation compared to ART 2340 plungers.
Results of break loose and glide force (BLG) performance are shown in Figures 5 - 6.
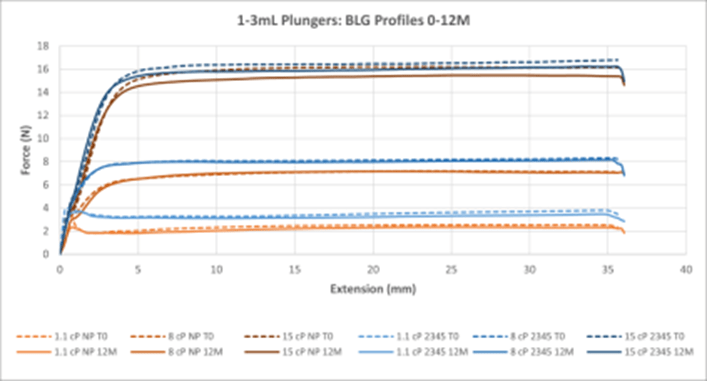
Figure 5. Summary of All BLG Data for 1-3mL NovaPure® and Legacy ART 2345 Plungers. Curves are averages of 30 samples at each timepoint.
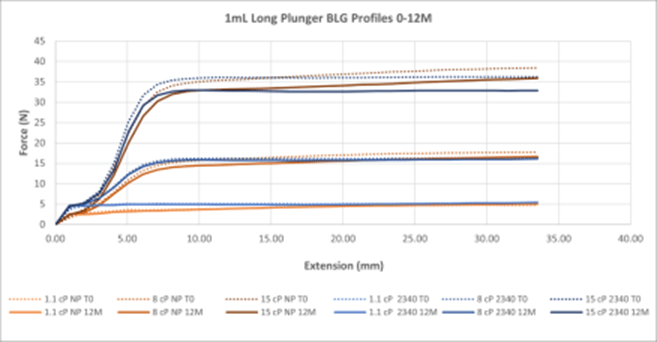
Figure 6. Summary of All BLG Data for 1mL Long NovaPure® and Legacy ART 2340 Plungers. Curves are averages of 30 samples at each timepoint.
Break Loose and Glide Force (BLG) Performance:
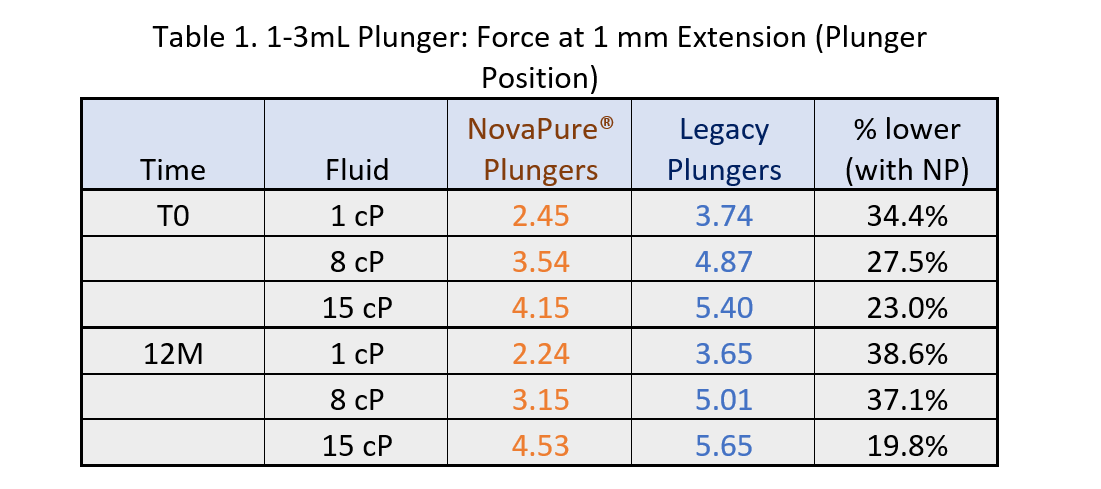
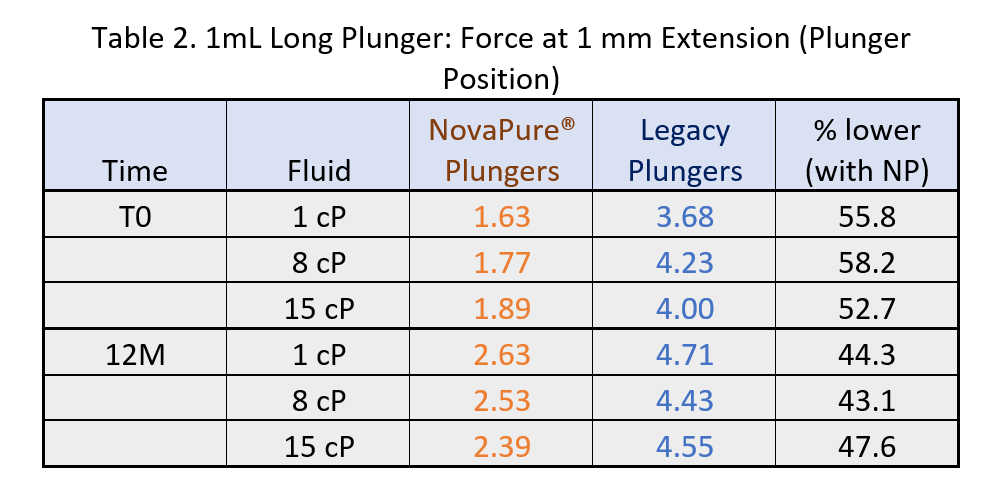
- BLG curves show that a lower break loose force is required by 1-3mL and 1mL Long NovaPure® plungers as compared to ART 2345 and ART 2340 plungers, respectively, and that this difference is more significant at lower viscosities (1.1 cP and 8 cP). See Tables 1 and 2.
- Both 1-3mL and 1mL Long NovaPure® plungers, as well as ART 2345 and ART 2340 plungers, show similarly constant glide forces (i.e. flat extrusion profiles) during extension (plunger position in mm in the syringe). Final forces required were higher for ART 2345 than 1-3mL NovaPure® plungers at all viscosities with the largest difference in final forces seen at 1.1 cP. 1-3mL and 1mL Long NovaPure® plungers had equivalent uniformity over time compared to ART 2345 and ART 2340 plungers, respectively.
- BLG performance was essentially the same between initial time 0 (dash lines) and 12 months (solid lines) for all plungers, showing consistency over time.
Results of break loose force at 1 mm extension are shown in Tables 1 - 2.
Work of Extrusion (Area under the Curve)
One way to quantify BLG performance is by calculating the work of extrusion, i.e., the energy required to move the plunger through the syringe barrel. Lower values are better. Work is defined as:
Work (Joules) = Force (Newtons) x Distance (meters)
Since a BLG curve plots force vs. extension (i.e., distance), the area under a BLG curve is work, i.e., work of extrusion or energy demand.
Figures 7 - 8 show work of extrusion.
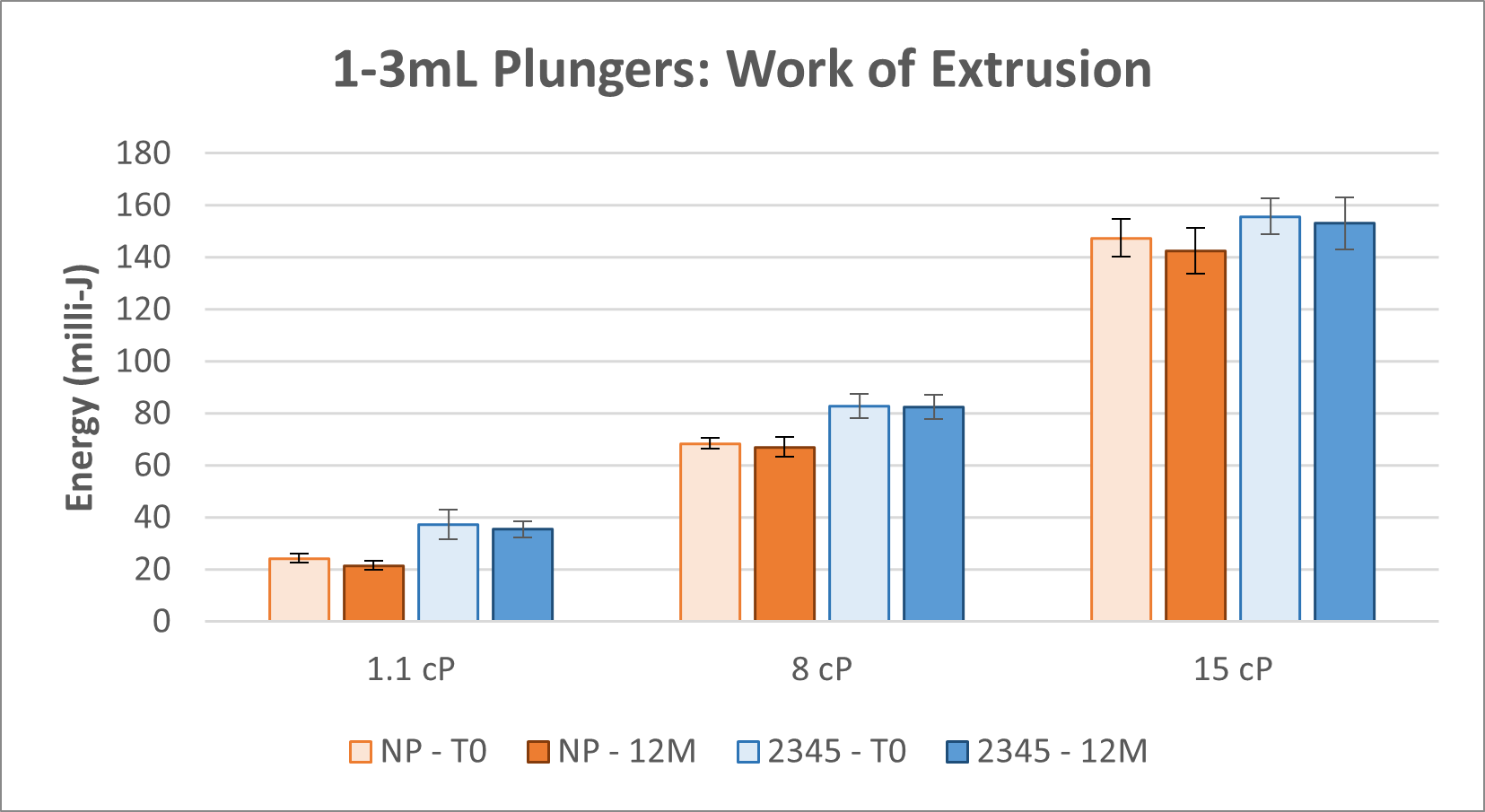
Figure 7. Work of Extrusion for 1-3mL NovaPure® and Legacy ART 2345 Plungers.
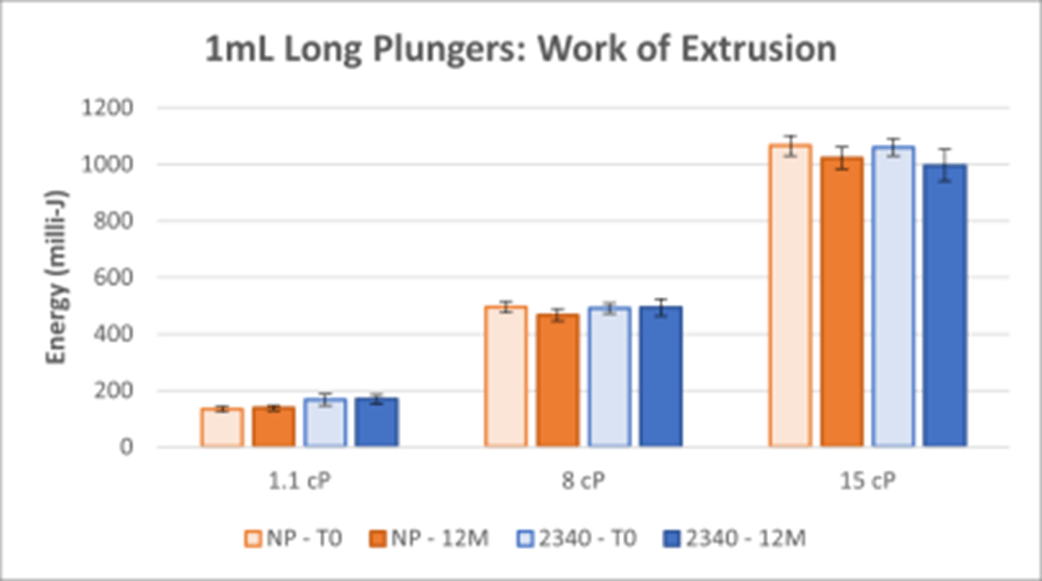
Figure 8. Work of Extrusion for 1mL Long NovaPure® and Legacy ART 2340 Plungers.
Work of Extrusion:
- Work of extrusion data show that 1-3mL NovaPure® plungers require less energy and have better reproducibility with the largest difference seen for 1.1 cP solutions compared to ART 2345 plungers. Work of extrusion data show that 1mL Long NovaPure® plungers are comparable to ART 2340 plungers in energy required and reproducibility.
Conclusions
NovaPure® plungers performed as well as or better than legacy plungers in all aspects tested. Of particular note, NovaPure® plungers show lower BLG and overall lower energy required than legacy plungers, which are valuable performance improvements for use in pre-filled syringe autoinjector systems. As drug product manufacturers look to extend the lifecycle of their drug products using more convenient delivery systems such as pre-filled syringe autoinjector systems, selection of quality components is essential to performance of autoinjector applications.
References:
West’s products are sold on the basis that it is the customer’s responsibility to evaluate and test the West product to determine its compatibility with other materials and fitness for any end use.West and the diamond logo, NovaPure® and FluroTec® are registered trademarks of West Pharmaceutical Services, Inc. in the United States and other jurisdictions. FluroTec® technology is licensed from Daikyo Seiko, Ltd.©2020 by West Pharmaceutical Services, Inc.All rights reserved. This material is protected by copyright. No part of it may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying or otherwise, without written permission of West Pharmaceutical Services, Inc.