Sealed for Safety: Understanding Injectable Drug Container Closure Systems
A container closure system (CCS) is comprised of packaging components, such as a vial and stopper, that together, contain and protect the drug product. There are many different packaging formats that you can select for your CCS, so it is important to have a thorough understanding of the various components included in the CCS, their required function, storage temperature and conditions, and the overall administration of the drug product.
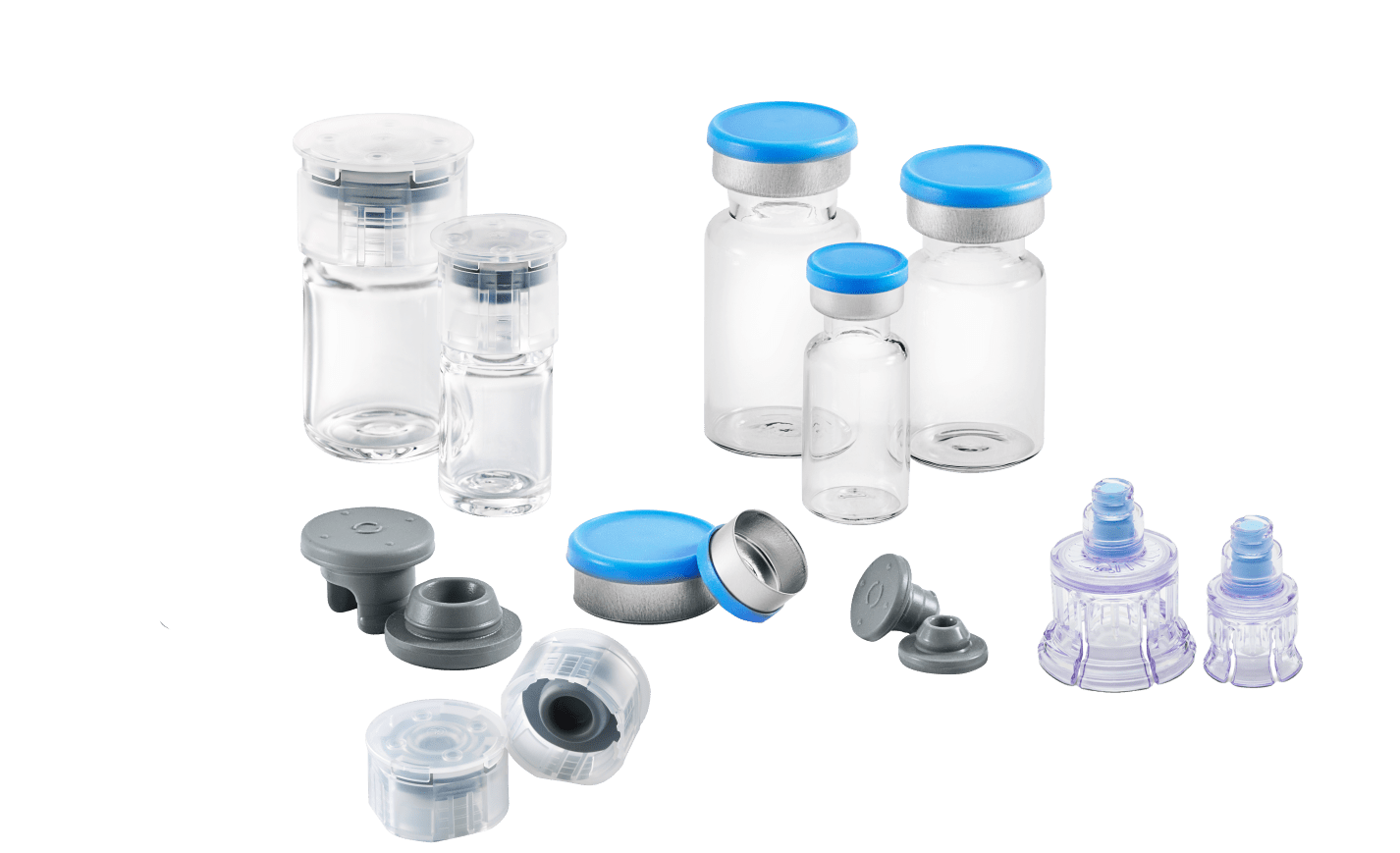
Vials:
Let’s start with the largest component by size: the container. This “container” is made from either glass or plastic, based on the application. Each material composition comes with its own advantages and disadvantages, so it is critical to understand the intended application for your drug product. The following breakdown helps in understanding why it matters.
Glass
- Borosilicate – used in conventional vials and has a high resistance to temperature change and chemical corrosion. It resists damage and cracking. Prone to delamination and extractables.
- Aluminosilicate – greater chemical resistance and higher operating temperature when compared to borosilicate glass. It offers a high resistance to breakage and damages as well as crack prevention. Eliminates delamination and has low extractables.
Through West’s collaboration with Corning Incorporated, West can support your drug filling with several glass vial options. Corning® Valor® glass vials are made from exceptionally strong aluminosilicate glass, developed to overcome some of the still-existing difficulties with the standard glasses on the market, which are delamination and glass breakage. Corning Viridian® vials are made from an optimized Type I borosilicate, reducing glass waste and manufacturing emissions , to deliver a more sustainable choice1.
Plastic
- Cyclic Olefin Polymers (COP) – a high performance polymer that is durable and has a higher impact resistance over Cyclic Olefin Copolymer (COC). These vials are resistant to acids , bases, and organic solvents and possess a higher heat deflection temperature over COC. COP vials are suitable for cold and cryogenic storage, making them ideal for cell and gene therapy applications.
- Cyclic Olefin Copolymer (COC) - a high performance polymer that is slightly less rigid than COP. More resistant to strong acids and oxidizing agents than COP. Offers high heat deflection temperature greater than 150°C.
West’s portfolio of vials also includes Daikyo Crystal Zenith® polymer vials. These cycle olefin polymer vials resist breakage and eliminate delamination risk. They are ideal for high-value drugs, cryogenic storage, and are demonstrated to contain advanced therapy drug applications such as gene therapies.
Stoppers:
The powerhouse of the CCS is the stopper, which is the primary seal of the system. This elastomeric component is essential in sealing the container closed, maintaining the sterility of the medication , allowing for withdrawal access, and then resealing for multi-dose applications. The stopper must allow for ease of needle or spike penetration as well as not cause elastomeric fragments that could cause needle clogging, or worse, be injected into the patient. Stoppers are categorized into two different categories:
- Serum – Primarily used for liquid drug applications. Used for single or multi-dose applications. Offers superb sealability after multiple penetrations. Stoppers are typically coated with a film to minimize packaging interactions with the drug product.
- Lyophilization – specifically designed for drugs that need to be lyophilized into a powder or cake for later reconstitution. Stoppers can be coated with a film to minimize packaging interactions with the drug product.
NovaPure® and Daikyo’s D-Sigma stoppers are two of the highest quality stopper product lines that West recommends from its extensive elastomer portfolio:
- NovaPure stoppers protect liquid and lyophilized drugs from visible and subvisible particles and unwanted stopper interactions.
- Daikyo D Sigma stoppers reduce variability with the tight particulate specification in the Daikyo portfolio, with 100%-dimensional verification.
Seals:
The stopper is held in place by the secondary closure, the seal. The seal can be either aluminum or plastic and holds the stopper against the flange of the vial. The seal exerts enough pressure around the stopper to create a sterile barrier from the drug inside and the exterior environment. It is imperative the seal maintains the pressure over the shelf life of the drug. The seal cannot warp, degrade or break based on the environment conditions or temperature and humidity. Seals are available in many designs, sizes and colors, ensuring that the material is unaltered prior to use and allowing for product identification. A few of the most popular seal types in the West portfolio include:
- Flip-Off® has an aluminum shell and a polypropylene button that is available in numerous colors. The button is flipped off by the users’ thumb prior to stopper access.
- Flip-Off® CCS (clean, certified, sterilized) also known as FOCCS seal is similar to a Flip-Off® seal, but they were designed to meet regulatory requirements for aseptic standards. They are provided ready-to-use with a specified low particulate and low bioburden levels.
- Daikyo PLASCAP® Closures - a one-step press-fit closure solution for serum applications, offering an alternative to aluminum seals
Drug Access Device Options
With the drug product safely contained, the CCS system can be accessed by several means to withdraw and administer the medication.
- Syringe and needle – Conventional gauged needle that pierces the septum of the stopper to access the drug product which is then withdrawn into the syringe for patient administration.
- Closed System Transfer Device (CSTD) – A vial adapter that provides a closed system to transfer liquids from the vial into a syringe to protect the patient and administer from harmful drugs or vapors.
- Vial Adapter™ – a device that pierces the stopper with a spike and attaches to the vial by grips that hold it in place. The adapter has a luer lock adapter so that a syringe can attach for drug withdrawal or reconstitution of the lyophilized drug product. It provides an open route for the transfer of liquids between the vial and a syringe. West offers several options for drug access and delivery.
Vials adapter transfer devices are becoming an increasingly popular drug access device. They allow for a single penetration, secure positioning, and easy access via a Luer lock syringe. There are several West designs that enable custom applications as well as patient administration.
- Vial Adapter™ transfer device - used in the transfer of drugs contained in the CCS . Designed with one spike and one lumen.
- Vented Vial Adapter™ transfer device - used in the transfer of drugs contained in a vial but is designed with one spike with two lumens and maintains pressure equilibrium by use of a 0.2-micron hydrophobic air filter.
- Swabable Vial Adapter™ transfer device - designed like the vial adapter above but has a valve that can be swabbed with alcohol to maintain sterility for multiple luer lock access withdrawals.*
- Mix2Vial® transfer device - a single use device optimized for lyophilized drugs. It is specifically made to connect two vials together and allows for Luer access after reconstitution by easily separating the two vial adapters.
- MixJect® transfer device - a single use device optimized for lyophilized drug reconstitution and administration by the pre-attached needle.
*When used in accordance with the Instructions for Use (IFU) and proper aseptic technique, microbial ingress is inhibited for up to 24 hours and 25 activations.
Summary:
This high-level overview was intended to provide you with a better understanding of the components that are available to create a customized system for the containment and delivery of your drug product. The design of your system must be assembled based on the drug product and its characteristics. There are many factors you need to consider when selecting your CCS such as:
- Is the drug chemical or biological and its constituents acidic, basic, volatile, hazardous, or viscous?
- What is the route of administration?
- At what environmental conditions will the drug be stored?
The selection of your drug packaging is important for the success of your launch as well as the patient safety and experience and should be considered an essential step you consider early on in your drug development.
Containment Solutions to Help you get Your Drug to Market:
The West Ready Pack™ containment solution is an ideal choice for a company in need of a proven CCS, combining high-quality ready-to use-components (stoppers, seals, and vials) that are designed to work together with demonstrated container closure integrity.
In the report “Characterization of Corning® Valor® Vials with West Vial Adapters” West has evaluated the Ready Pack components- NovaPure stoppers, FOCCS seals, and Corning Valor vials, together with West’s Vial Adapter transfer devices to show that West components are compatible and effective in meeting today’s stringent pharmaceutical drug delivery challenges.
You can learn more about these container closure components by visiting the Ready Pack containment solution web page and the Vial Adapter™ transfer device web page. If you would like to learn more about this Technical Report or about solutions and support that West can provide you in making your container closure selection, we invite you to Contact Us so that we can connect you with an account manager in your region.
Sources:
120% less glass and 30% less emission is based on 30% reduction in wall thickness. Results based on 3rd party Life Cycle Assessment of Corning Pharmaceutical Technologies conducted by Sphera Solutions Inc.
Important Product InformationRx Only.
Intended for US healthcare professionals.Vial Adapter™, Vented Vial Adapter™, and Swabable Vial Adapter™ transfer devices carry the CE mark (0344) and are 510(k) cleared by the United States Food and Drug Administration. Products are shown for INFORMATION purposes only and may not be approved for marketing in specific regions. Distribution and use are subject to applicable regulatory approvals and requirements for medical devices. The Vial Adapter and Vented Vial Adapter transfer devices are configurable and may not be suitable for use with all drugs. The Swabable Vial Adapter transfer device inhibits microbial ingress for up to 24 hours when used in accordance with the product instructions for use and proper aseptic technique. The use of the Swabable Vial Adapter transfer device should not be interpreted as modifying, extending, or superseding a drug manufacturer's labeling recommendations for storage and expiration dating. The Swabable Vial Adapter transfer device is not compatible for use with all drug products. Refer to drug manufacturer's labeling and use instructions for device configuration compatibility and for shelf life and sterility information of opened or reconstituted drug product. Failure to follow product instructions for use may result in compromised sterility; contamination; leakage (including possible exposure to medication); and/or inadequate medication reconstitution, transfer, and/or dosing. Product misuse could potentially lead to user and/or patient infection, suboptimal therapy, and/or delay in therapy. Please contact your West Pharmaceutical Services, Inc. (West) representative for product availability. Important product and safety information and warnings for Vial Adapter, Vented Vial Adapter, and Swabable Vial Adapter transfer devices, respectively, at:
https://www.westpharma.com/-/media/WestPharma/Files/Products/Vial-Adapter-Indications-Safety-and-Warnings.pdf,
https://www.westpharma.com/-/media/westpharma/files/products/vented-vial-adapter-indication-safety-and-warnings.pdf, and
https://www.westpharma.com/-/media/westpharma/files/products/swabable-vial-adapter-indications-safety-and-warnings.pdf
The Mix2Vial® transfer device is 510(k) cleared by the United States Food and Drug Administration and carries the CE mark (0344). Products are shown for INFORMATION purposes only and may not be approved for marketing in specific regions. Distribution and use are subject to applicable regulatory approvals and requirements for medical devices. Please contact your West Pharmaceutical Services, Inc. (West) representative for product availability. Important product and safety information and warnings at:
https://www.westpharma.com/-/media/westpharma/files/products/mix2vial-indication-safety-and-warnings.pdf.
The MixJect® device is 510(k) cleared by the United States Food and Drug Administration and carries the CE mark (0344). Products are shown for INFORMATION purposes only and may not be approved for marketing in specific regions. Distribution and use are subject to applicable regulatory approvals and requirements for medical devices. The MixJect® device is configurable and may not be suitable for use with all drugs. The device may contain a pre-attached needle containing cobalt. Please contact your West Pharmaceutical Services, inc. (West) representative for product availability. Important product and safety information and warnings at:
https://www.westpharma.com/-/media/westpharma/files/products/mixject-indication-safety-and-warnings.pdf.
NovaPure, Flip-Off, and Ready Pack are trademarks or registered trademarks of West Pharmaceutical Services, Inc. in the United States and other jurisdictions.
MixJect, Mix2Vial, Vial Adapter, Swabable Vial Adapter, and Vented Vial Adapter are trademarks or registered trademarks of West Pharma. Services IL, Ltd., a subsidiary of West Pharmaceutical Services, Inc.
Crystal Zenith, D Sigma, and PLASCAP are trademarks of Daikyo Seiko, Ltd.
Corning, Valor, and Viridian are trademarks of Corning Incorporated.
Crystal Zenith, D Sigma, and PLASCAP technologies are licensed from Daikyo Seiko, Ltd.
West Pharmaceutical Services, Inc. is the exclusive distributor of Corning Valor glass vials.



