Solving the Challenges of Storage and Delivery of Gene Therapies
Development in gene therapies is continually increasing – presently over 1,400 companies are involved, with over 2,200 clinical trials underway. It is essential to find an appropriate containment system to protect the time and monetary investments required to create these treatments.
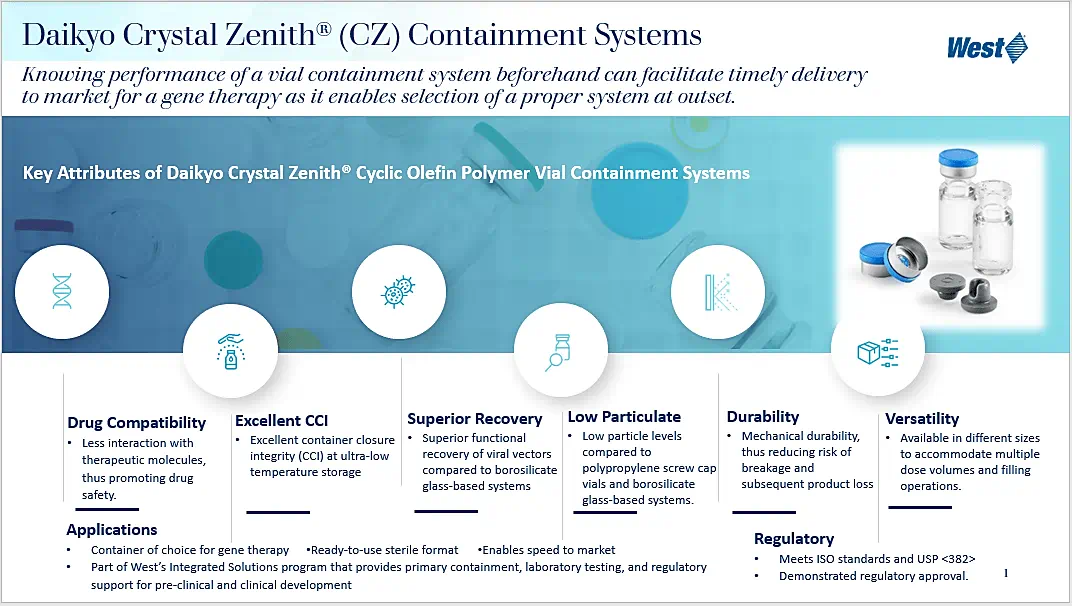
A challenge for storage and delivery of gene therapies, which is not encountered with most other drug products, is the need to maintain efficacy through storage at ultra-low temperature (ca. -80°C). Understanding this, over the past several years we have undertaken numerous studies to evaluate the suitability of our packaging components. The recently published e-book, Vial Containment Systems for Gene Therapies, summarizes this work, characterizing the performance of containment systems comprising Daikyo Crystal Zenith® (CZ) cyclic olefin polymer vials and halobutyl elastomer stoppers with FluroTec™ poly(ethene tetrafluoroethene) laminate film facing drug product. Comparison is made to systems based upon borosilicate glass and polypropylene. The chapters focus on:
- Fundamentals and commercial impact of gene therapies,
- Performance of FluroTec™ film and CZ vials in reducing interaction with drug product, and reducing migration of compounds from the stopper,
- Intrinsic benefits of West components from standpoints such as coefficient thermal expansion match, mechanical durability, and particle levels,
- Container closure integrity through two years at -80°C, and
- Maintenance of viability for adeno-associated viruses, adenoviruses, and lentiviruses.
When all factors are considered, the performance of the West system exceeds or matches the performance of borosilicate glass and polypropylene based systems. Knowing the performance of a vial containment system in advance facilitates proper selection and ultimately timely delivery to the market for gene therapy.
Choosing the Best Vial for Your Viral-Vector Drug Product
Viral vectors must be stored at temperatures close to -80°C, so it is vital to select a vial-stopper-seal combination which preserves the viral vectors at ultra-low temperatures. A cyclic olefin polymer (COP) vial, combined with an ETFE-coated halobutyl elastomer stopper, may be ideal for preserving viral vectors in cryogenic conditions. West conducted a multi-year study using the following components:
- Daikyo Crystal Zenith® (COP) vials
- NovaPure® stoppers (bromobutyl) with FluroTec (ETFE) film
- Daikyo Seiko’s elastomer (bromobutyl) serum stoppers with Flurotec™ (ETFE) film
- Flip-Off® seals
These vial-stopper-seal combinations were shown to be preferrable to drug containment systems using glass vials, or uncoated stoppers, in the areas of container closure integrity (CCI), particle levels, interaction with protein-based drug products, breakage, and protein recovery.
Container Closure Integrity
Container Closure Integrity (CCI) is optimized by using vials and stoppers with similar Coefficients of Thermal Expansion (CTEs), meaning their volumes are reduced to roughly the same degree during refrigeration. A CZ vial and a bromobutyl stopper shrink in a similar manner under low and ultra-low temperature conditions. Borosilicate glass, by contrast, retains its volume when refrigerated. When paired with an elastomer stopper, glass may be separated from the stopper when the stopper shrinks, but the glass vial does not. The resulting compromised CCI could lead to contamination or loss of the contained drug product. Table 1 shows the vast difference in percentage of volume reduction in glass at ultra-low temperatures versus CZ and elastomers.
Table 1. Coefficients of Thermal Expansion and Volume Changes (1,2)| Material | Coefficient of Thermal Expansion
(10⁻⁶ cm/cm·K) | Volume Reduction (%)
(25°C → -80°C) |
|---|
| Borosilicate glass | 4 | 0.12% |
| Typical elastomer | 77 | 2.4% |
| CZ | 70 | 2.2% |
Particle Levels
Patient safety can be compromised by particle contamination of a parenteral drug. Various sizes of particles threaten different organs: large particles can damage veins, smaller particles may clog capillaries, and tiny particles can damage any organ to which blood flows.3 Particles in glass vials are often the result of delamination, or the shedding of tiny vial glass particles from the vial surface. Particle levels can be greatly reduced by using COP (CZ) vials instead of borosilicate glass vials.
Reduced Interaction with Protein-based Drug Products
The potential for interactions between protein-based drug products and elastomeric stoppers can be drastically reduced by the addition of a fluoropolymer, such as FluroTec barrier film, to the drug-product side of the stopper. This film both reduces the surface energy of the elastomeric material and creates a barrier between the stopper and the drug product to reduce protein-elastomer interactions. In one study, West evaluated the interactions of three types of protein with uncoated elastomer stoppers and FluroTec barrier film-coated stoppers by quantitative comparisons of particle levels and turbidity. The study showed that FluroTec film greatly reduced the number of particles and lowered the turbidity for all three types of protein.
Mechanical Durability
Crystal Zenith vials were compared to glass vials from several manufacturers and were found to be more resistant to fracturing than glass vials when force is applied both vertically and horizontally.
Protein Recovery
When protein-based drug products, such as gene therapies, are stored in CZ vials capped with FluroTec film-laminated stoppers, there is a reduced risk of proteins adsorbing into the elastomer or the vial. Adsorption of the viral vector into either the glass vial or the uncoated stopper would decrease the potency and effectiveness of an injection from a compromised vial.4 West tested four protein types for interactions with uncoated and FluroTec film-laminated stoppers by shaking the solutions for 24 hours before using size exclusion HPLC to measure protein recovery. All four showed higher recovery in the vials with the FluroTec film-laminated stoppers.
In another study, both CZ and polypropylene (PP) vials were shown to preserve close to 100% protein recovery, whereas glass vials had a markedly decreased protein recovery.
Conclusion
Careful consideration must be put into selecting the best vial-stopper-seal containment system for each gene therapy drug. As cold storage is mandatory for many of these drugs, CCI must be considered at every stage of the freezing and thawing process. Per West studies, the best option may be a cyclic olefin polymer vial combined with a fluoropolymer-coated stopper. For more detailed information and study data, please reference the eBook Vial Containment Systems for Gene Therapies
Crystal Zenith is a trademark of Daikyo Seiko, Ltd., and CZ and FluroTec technologies are licensed by Daikyo Seiko.
References
1. http://www.engineeringtoolbox.com/linear-expansion-coefficients-d_95.html (accessed 4/19/23)
2. Characteristics of Daikyo Resin CZ. Daikyo Seiko, Ltd. Technical Report DS-CZ-E017 (January 2022)
3. West Technical Report CZ TR 2022-038 Comparison of Particles in Daikyo Crystal Zenith® Vials, Glass Vials, Polypropylene Screw Top Vials and Cryogenic Bags
4. West Technical Report CZ TR 2020/030 Evaluation of Daikyo Crystal Zenith® Vials for the Cryopreservation of Therapeutically Relevant Human Cell Types