Part II - Daikyo Crystal Zenith Polymer How does it compare to other packaging options
This is the second in a two part series dedicated to the origins and attributes of the Daikyo Crystal Zenith® polymer
![]()
The Daikyo Crystal Zenith® polymer is a custom formulated cyclic olefin polymer developed by Masamichi Sudo, who made it his life’s work to improve all aspects of parenteral packaging. He started by creating safe closure solutions like B2 lubricious coating and FluroTec®, a fluoropolymer lamination to protect both elastomer and drug product from each other. Enormously successful, these technologies paved the way toward the creation of the Daikyo Crystal Zenith (CZ) polymer.
Probably the most salient feature of CZ is its outstanding barrier properties. CZ outperforms many plastics with respect to this important parameter. One of the most deleterious gases to parenteral pharmaceutical products is oxygen. Many drug products are subject to oxidative reduction and therefore must be protected from oxygen throughout their shelf life. CZ has better oxygen permeability properties than many of its peer polymers such as polypropylene, polystyrene and polycarbonate.
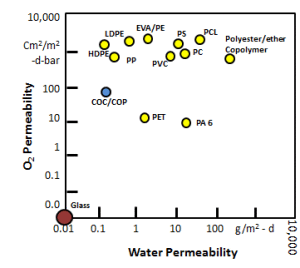
In addition to good oxygen barrier, CZ provides protection against the next most deleterious material to many drug products: water vapor. This is a plus for manufacturers that are packaging non-aqueous and dry drug products.
Several studies have been performed that indicate CZ is a good candidate for moisture sensitive products. This property is inherent to CZ thanks to its nonpolar material properties.
The next area of concern for packaging scientists is leachables and extractables. While CZ is free of inorganic extractables (unlike glass), how does it stack up against its peer polymeric packaging materials? Data indicates CZ has low organic extractables especially when compared to other plastic packaging materials.
Finally, it helps to be sure that the selected packaging materials do not selectively aggregate, sequester, absorb or adsorb the important active and excipient material in the drug product. It is well known that some plastics can completely remove some excipients from the drug product, the result being instability and shorter shelf life.
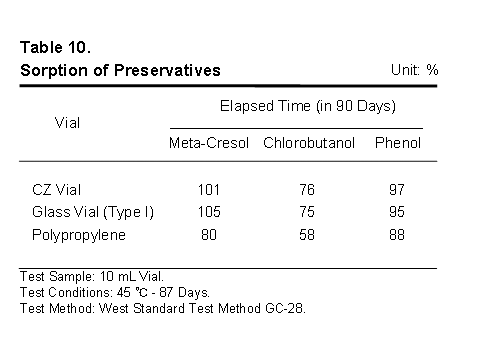
This table details the amounts of three widely used preservatives measured in solution after 90 days of storage. Values over 100 percent indicate experimental error. Note CZ is equivalent to glass and superior to polypropylene in regard to sorption of these model preservatives.
A CZ system can mitigate some of the many issues pharmaceutical manufacturers may encounter with current packaging. These companies have their hands full with the research, development, clinical trials, regulatory submissions, marketing and production of the drug product. By working with West, researchers are able to leverage our expertise with primary containers and focus on the drug product itself.




