Studies Demonstrate Potential Benefits of Intradermal Injection
In a previous blog post, we discussed various devices and solutions used to overcome the challenges associated with Intradermal (ID) injections. These challenges are mainly associated with the Mantoux Technique, a manual ID injection using a needle and syringe. Lacking the skill needed for this technique creates a risk of injecting the product too deep or not sufficiently deep and rendering the dose either less or potentially ineffective. Previous posts also touched on some of the potential benefits associated with ID delivery when compared to the more familiar Subcutaneous or Intramuscular type injections. Two of the benefits we look at closer in this post are dose sparing and improved immune response. Additionally, we will see how these two benefits can be related.
![]()
In a study conducted by NanoPass Technologies Ltd. and Crucell Holland B.V. titled "Clinical Evaluation of a Novel Microneedle Device for Intradermal Delivery of an Influenza Vaccine: Are All Delivery Methods the Same?"1, it was concluded that the route of administration (ID vs. IM) as well as the delivery method (NanoPass MicronJet™ device vs. regular needle and syringe [Mantoux technique]), can result in a significantly higher antibody response. In addition, the data showed that using a fractionated dose by ID produced the same immune response to that of full dose IM. The below graph show antibody titers to the 3 strains included in the particular influenza A vaccine tested.
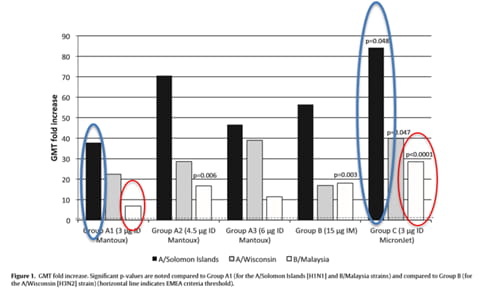
Immunogenicity (Fig. 1 and Table 2) was higher in the 3 g ID group using the MicronJet needle (Group C) compared to the ID groups using the conventional Mantoux needle (Groups A1, A2, and A3) and the IM group (Group B).
The observed difference in immunogenicity is possibly due to a higher success rate of delivering the vaccine to the dermis, where there is a richness in antigen presenting cells. Furthermore, the intradermal route via MicronJet microneedle may be viable for those that are low or even non-responders of the vaccine, as it shows a higher response in regards in to Geometric Mean Titer (GMT) fold increase that can possibly be correlated to a better immune response.
The full dose of 15 micrograms of the vaccine delivered via IM (Group B) shows no statistical difference when compared to 3 micrograms of the vaccine via MicronJet microneedle (Group C). This indicates that the vaccine administered with the MicronJet microneedle will reach the same immune response at a fraction of the dose (20%) of the full IM.
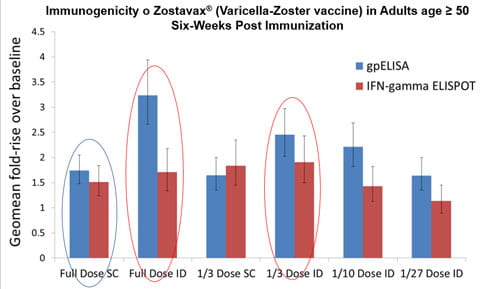
A second study, "Immune Response and Reactogenicity of Intradermal Administration Versus Subcutaneous Administration of Varicella-zoster Virus Vaccine: An Exploratory, Randomized, Partly Blinded Trial”2, focuses on the zoster vaccine, Zostavax®, that is commonly administered subcutaneously (SC) to prevent shingles. The graph depicts that participants who received full dose intradermal and 1/3 dose intradermal vaccination had significantly higher Geometric mean fold rise GMFR (p<0·0001) in varicella-zoster virus antibody titres post-vaccination than did those who received full subcutaneous dose. The lower intradermal zoster vaccine doses induced GMFRs comparable to that of full dose zoster vaccine given subcutaneously.
West now offers two unique devices designed to help deliver a reliable and consistent ID injection each and every time. The ID Adapter is a snap on guiding device ideal for mass vaccination in developing regions. West's recent partnership with NanoPass Technologies, Ltd provides a microneedle solution, MicronJet600™, which is ideal for a variety of immunotherapies and vaccinations. Both devices are suitable for a broad range of ages.
Contact West today to learn more about how we can help you overcome the ID delivery challenge.
MicronJet™ and MicronJet600™ are trademarks of NanoPass Technologies, Ltd.
Zostavax® is a registered trademark of Merck Sharp & Dohme Corp.






