West’s Advanced Elastomer: 4040/40 - Created with Insight to Power the Future
In its 95+ year history, West has developed and marketed a very large number of elastomeric formulations, each designed to address particular market demands. Over this time, various aspects of drug product quality have gained attention – a fact reflected in the numerous regulatory guidances that have been issued. An early focus of these guidances was extractables and leachables, followed closely by particulate matter and container closure integrity. Beyond these regulatory requirements, the market demands product portfolios that enable platforming and predictable, consistent product quality and performance.
![]()

West is addressing these regulatory and market requirements through a new elastomer platform. The West 4040/40 elastomer platform has been developed using the quality-by-design (QbD) approach. A predefined quality target product profile (QTPP) that emphasizes product and process understanding was used to evaluate all potential raw materials. Initial selection of raw materials leveraged historic knowledge of formulation chemistry. Further, the raw materials’ chemical/physical properties were critically analyzed to identify alternate sources, should they be required.
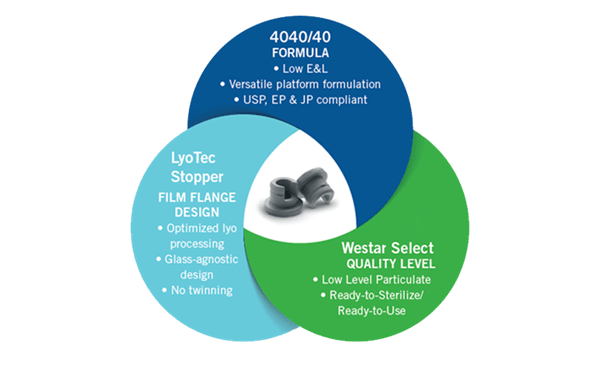
Using the QbD approach, the 4040 elastomer platform delivers in the key areas of:
- Extractables: in-depth raw materials profiles were established to satisfy the QTPP and identify residual risks to safety and performance. Comprehensive extractable evaluations were conducted and linked back to the raw materials.
- Particulate matter: careful selection of raw materials and optimization of the formulation minimizes the introduction of high levels of intrinsic particulate matter (> 25µm). The risk of particulate is further mitigated through the Westar® wash process.
The breadth and depth of documented knowledge for the 4040/40 elastomer sets it apart from any historic elastomer platforms in the market. The knowledge space around the 4040/40 elastomer platform supports the increased demands of Knowledge Management by regulators. Developing high performing elastomers/components is just one example of West's commitment to the safe and efficient delivery of drug products to patients.
Click here for more information on the 4040 LyoTec® stopper.
Westar® and LyoTec® are registered trademarks of West Pharmaceutical Services, Inc., in the United States and other jurisdictions.




