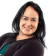Vial Containment Systems for Gene and Cell Therapies
Cell and gene therapies are an expanding area with an expected growth rate of 24.7% over the forecast period of 2022-2030 .1
Vial-stopper-seal combinations used as containment systems for these therapies need to maintain container closure integrity (CCI) for extended periods. Achieving CCI can be a particular challenge due to the low storage temperatures required: ultra-low (-80°C) for gene therapies and cryogenic (≤ -130°C, i.e., vapor of liquid nitrogen) for cell therapies.

To determine suitability for gene and cell therapies, West evaluated two vial-stopper-seal combinations based on: Daikyo Crystal Zenith® (CZ) cyclic olefin polymer vials – fluoropolymer (ethylene tetrafluoroethylene)-laminated elastomer stoppers – Flip-Off® CCS seals. Evaluation of CCI was at ultra-low (-80°C) and cryogenic (≤ - 130°C) temperatures.
The following sterilized components, which are available in the Ready Pack™ containment solution, were used:
- Daikyo Crystal Zenith® (CZ) cyclic olefin polymer vials (2 mL)
- 13 mm Serum NovaPure® (NP) stoppers RP ART 1358 4023/50G
- 13 mm Serum Daikyo Seiko, Ltd. stoppers S2-F451 D21-7S RB2-40 RUV
- Flip-Off® CCS (clean, certified, sterilized) seals 13 mm CCS Seal 5922-1120
These components were selected for evaluation based on their ability, among the West portfolio, to present the lowest risk to drug product performance.
Elastomer stoppers laminated with fluoropolymer barrier films are known to be less interactive with selected protein-based molecules than non-laminated elastomer stoppers, based on prior West research.2,3
Polymer vials, such as CZ vials, have intrinsically less interaction with molecules than glass vials, based upon lower surface energy. In prior West research, this has been demonstrated; selected protein-based molecules showed less interaction with CZ vials than with glass vials.4,5 Further, typical elastomer and cyclic olefin polymer have a closer match in coefficient of thermal expansion than typical elastomer and glass.6 Glass and elastomers shrink at different rates as temperatures are lowered, creating the possibility of a breach in the stopper-vial-seal combination. However, CZ vials tend to shrink at the same rate as stoppers, maintaining better level of contact throughout the process and reducing risk of a breach.
A two-year CCI study now has been completed examining systems comprising CZ vials, NovaPure® and Daikyo Seiko fluoropolymer-laminated stoppers, and Flip-Off® CCS seals at -80°C (refrigerator) and ≤ -130°C (dewar with vapor of liquid nitrogen). West validated methods employing tracer gas leak detection with helium (He leak) and gas headspace analysis with O2 were used. Both methods are derived from USP Chapter <1207>.
The results of this study have just been published:
- Long-Term Container Closure Integrity Testing of Vial-Stopper-Seal Combinations Comprising Daikyo Crystal Zenith® Vials at Ultra-Low and Cryogenic Temperatures. West Pharmaceuticals Services, Inc. Technical Report 2023/261 and presented by Amy Gindhart (Manager, Scientific Communications):
- Strategies to Evaluate CCI of Vial and Syringe Systems over Time and Temperature. 2023 PDA Parenteral Packaging Conference (April 19, 2023)
For all systems, He leak testing verified vial/stopper seal integrity over 2 years at room temperature, -80°C and ≤ -130°C. More critical, though, O2 headspace analysis demonstrated good CCI with no system failure over 2 years at -80°C and ≤ -130°C. See Figures 1 and 2.At ≤ -130°C, no N2 ingress was observed over 2 years. At -80°C, no O2 ingress was observed after 1 year and only slight O2 ingress (5%) was observed after 2 years – most likely from slow diffusion through CZ. Note there was no breach of any sample at -80oC, as that would have resulted in 21% O2 (i.e., atmosphere of refrigerator).
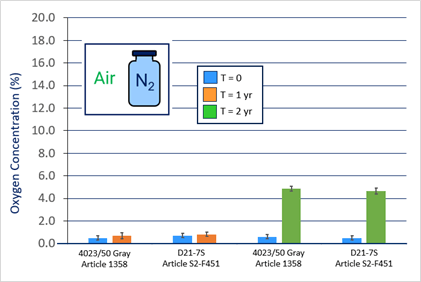
Figure 1. Oxygen Headspace at -80°C.
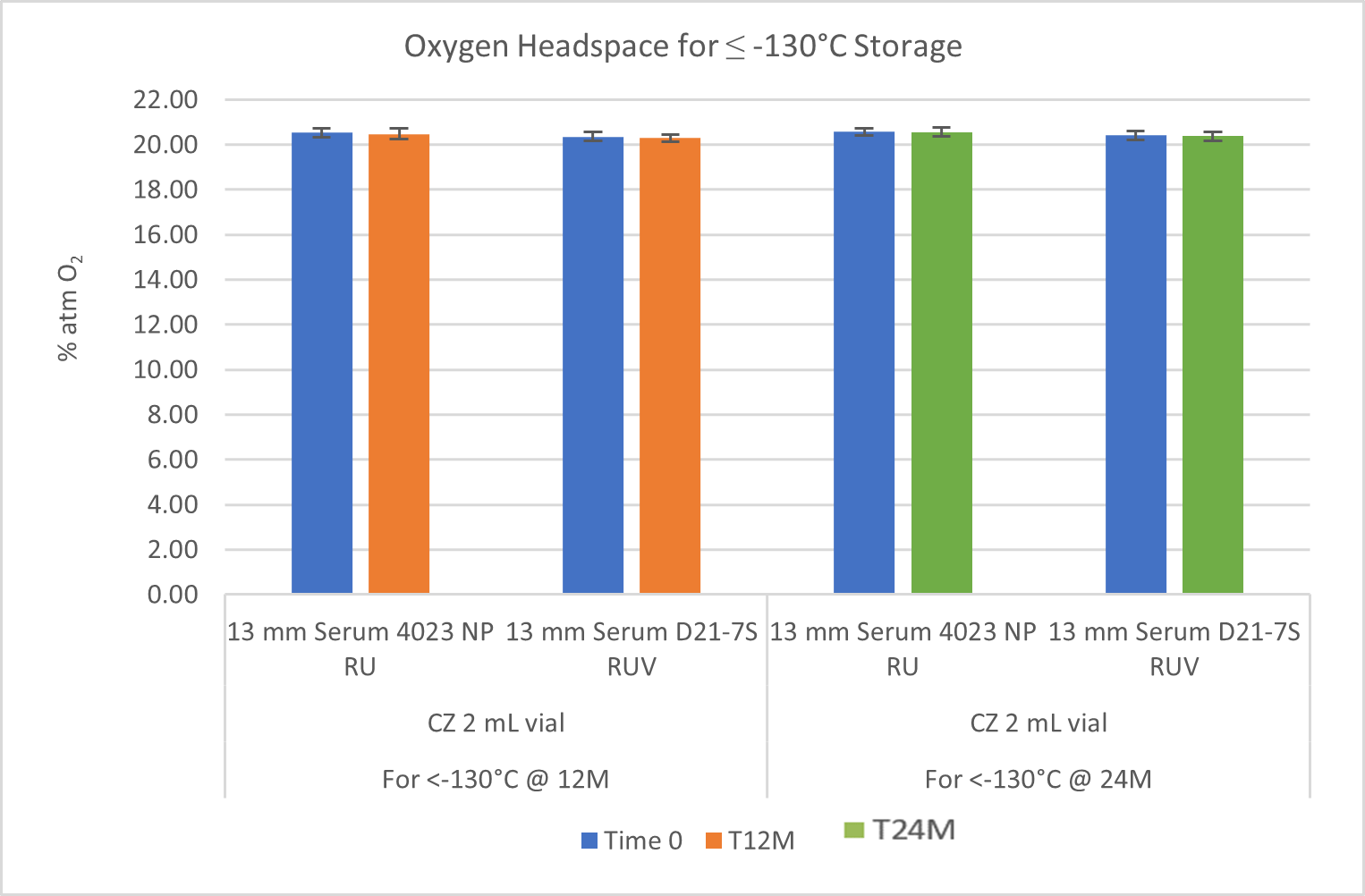
Figure 2. Oxygen Headspace at ≤ -130°C.
At ≤ -130°C, no N2 ingress was observed over 2 years. At -80°C, no O2 ingress was observed after 1 year and only slight O2 ingress (5%) was observed after 2 years – most likely from slow diffusion through CZ. Note there was no breach of any sample at -80oC, as that would have resulted in 21% O2 (i.e., atmosphere of refrigerator).
Based just upon physio-chemical properties, vial-stopper-seal containment systems based on CZ vials and fluoropolymer-laminated elastomer stoppers have the potential to be suitable for gene and cell therapy drug products.
The above studies indicate CZ vial-stopper-seal containment systems can likely provide adequate physical protection and stability of the cell and gene therapy product maintaining the integrity of the drug product throughout manufacturing, storage, and shipment.
In addition, the containment system offerings can work in the manufacturing process and be sufficiently scalable for commercial success.
Visit our Ready Pack™ containment solutions page to learn more about the components mentioned or for more information on product offerings, please contact a Technical Customer Service (TCS) representative or Account Manager.
References:
- Compiled from information available at: https://www.coherentmarketinsights.com/market-insight/cell-and-gene-therapy-market-2475 (accessed 7/26/23)
- R. Singh, L. Waxman, L. Fang, C. Zhao. An Investigation to Examine the Effect of the Elastomeric Surface Treatment on Protein Stability. PDA Journal of Pharmaceutical Science and Technology, 75, 230-244 (2021)
- R. Singh, W. Garzon-Rodriguez, C. Zhao, P. Frandolig, P. McAndrew. Elastomer Stoppers with FluroTec® Film: The Right Choice for SARS-CoV-2 Vaccines (published online – May 22,2020) https://www.westpharma.com/en/blog/2020/May/sars-cov2-vaccines-primary-packaging-challenges
- R. Singh and L. Waxman. Effect of Container Surface on Adsorption and Aggregation of Protein Therapeutics. 2018 PDA Universe of Pre-Filled Syringes and Injection Devices, Orlando, FL (August 8, 2018) https://www.westpharma.com/Site-Repository/Knowledge-Articles/Registered/ka-01531
- D. DeCou, W. Garzon-Rodriguez, M. Gehrmann, P. Frandolig, P. McAndrew. Alternative for SARS-CoV-2 Vaccine Primary Package Systems: Daikyo Crystal Zenith® Cyclic Olefin Polymer Vials (published online – September 2, 2020)a. https://www.westpharma.com/en/blog/2020/September/sars-cov-2-vaccines-alternative-vial-components
- M. Gehrmann, O. Laskina, L. Fang, P. McAndrew. Challenges in Low-Temperature Storage of Cell Therapy Drug Products. PepTalk 2020, San Diego, CA, January 20, 2020
This document is for informational purposes only. West’s products and solutions are sold on the basis that it is the customer’s responsibility to evaluate and test the West product or solution to determine its compatibility with other materials and fitness for any end use. WEST MAKES NO WARRANTIES, WHETHER EXPRESS, IMPLIED OR STATUTORY, INCLUDING, WITHOUT LIMITATION, THE WARRANTIES OF MERCHANTABILITY AND FITNESS FOR PARTICULAR PURPOSE, RELATING TO THE INFORMATION IN THIS DOCUMENT.
NovaPure® and Flip-Off® are registered trademarks of West Pharmaceutical Services, Inc. in the United States and other jurisdictions.
Crystal Zenith® is a registered trademark of Daikyo Seiko, Ltd.
Crystal Zenith® technology is licensed from Daikyo Seiko, Ltd.