Inspecting Elastomer Components: Can You Spot a Defect?
Spotting a quality issue on a stopper or plunger can be difficult. While a trimming or molding defect may be noticeable, embedded particulate or foreign contamination may be far too small for the human eye to see.
![]()
Take for example, the image of the plunger below. At actual size, which is about as big as a fingernail, would you be able to spot the defect?
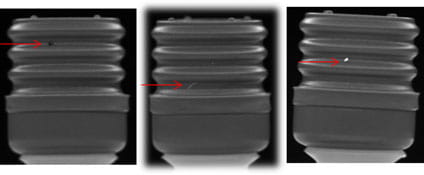
Vision verification is an established and easy way of improving the quality of pharmaceutical components. Through the use of automatic, program-controlled vision inspection technology, all surfaces of a component can be quickly and easily verified – helping to increase product yield by reducing the number of rejects. Automated inspection also can help to reduce waste and increase operational efficiency by optimizing throughput.
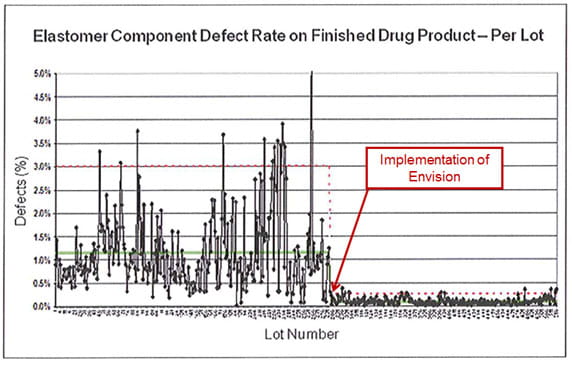
The above image shows how the use of vision verification may help to make notable improvements in consistency and yield rates.
West is a pioneer in vision inspection technology. The Envision® automated inspection system can enhance the quality of Westar® ready-to-sterilize components by significantly reducing adhered and embedded particulate, helping to improve consistency.
Many of our standard products are available in Envision-verified formats – an upgrade that can be made simply by talking to your sales representative as there’s no need for a complex development agreement or even additional regulatory work. Your current item may already be available with Envision verification, allowing you to benefit from the advantages of a tighter specification and reduced end of line component failure risks while preserving all the features of your current formulation, configuration, finishing, packaging and other key details. This approach to upgrade, which leaves the fundamentals of your component’s production and preparation unchanged, is not associated with any additional testing or regulatory reporting work.
Contact a West Technical Customer Service representative today to see if your products are pre-qualified as Envision-verified components.
Envision® and Westar® are registered trademarks of West Pharmaceutical Services, Inc., in the United States and other jurisdictions.




