Industry Insight on Container Closure Integrity
The regulatory environment is moving to a more science-based approach to Container Closure Integrity (CCI). This is driven by the recent publication of United States Pharmacopeia Chapter <1207> Package Integrity Evaluation – Sterile Products. Recently, West gave a webinar (377 registrants) titled Container Closure Integrity: Six Keys to Simplify Your Strategy and Execution, that addressed Chapter <1207> and other topics. The survey questions (over 100 responses) from this webinar are instructive.
![]()
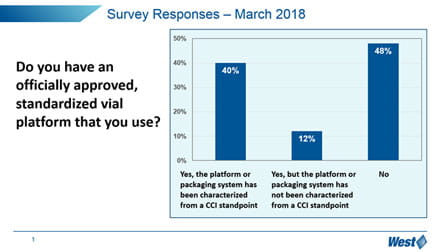
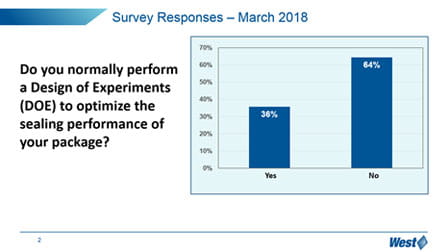
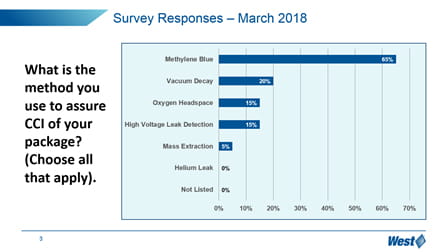
It is clear that the market, in general, is still trying to understand how to manage these new expectations, and manage the optimization and qualification of vial containment systems. In particular, “change management” is a challenge – 65% of respondents have not upgraded to the more sensitive and deterministic analytical techniques promoted in Chapter <1207>, but still reply upon probabilistic methylene blue dye ingress.
In order to achieve a better understanding to address these challenges, and others in CCI, see A Holistic Strategy for Container Closure Integrity: Selecting and Evaluating an Integral Packaging System, PDA Journal of Pharmaceutical Science and Technology 72 (1), 15-34 (January-February 2018). Visit the West Knowledge Center, which houses various materials on the subject, or contact a Technical Customer Support representative for more information.





