Aluminum Seals with Plastic Buttons
Aluminum seals with plastic buttons, such as West Flip-Off® Seals (FOS) and
West Flip-Off Tear Off Seals (FOTOS) allow for one-handed opening. Available color-coding on buttons enhances drug identification and market differentiation.1 They provide tamper evidence (i.e., once the button is removed, it cannot be re-attached properly). Additionally, West seals are specially manufactured with a tapered aluminum shell design; there is no risk of exposed sharp edges, and concomitant risk of injury, due to bridge lift-up after button is removed. They are most commonly used for injectable drug products.
Between FOS and FOTOS, FOS are more commonly used for injectable drug products. FOTOS are used usually for special applications where there is a need for complete removal of the seal and stopper to access the vial content.
Flip-Off® Seal Mechanisms: Bridge Versus Controlled Score
A key feature of both FOS and FOTOS is a bridge, which is a small connecting strip of aluminum that secures the plastic button to the aluminum shell. The bridge breaks when the plastic button is removed. Another approach to removing a plastic button is called controlled score. This comprises a design such that the seal is indented to a prescribed thickness. The indented area is where the fracture will occur when the plastic button is removed. This concept is similar to a soda can opening.
West recommends use of bridges, instead of the controlled score approach. Bridges are more forgiving in the crimping process and have lower variation in removal force, as compared to inflexible controlled scores. Seals with bridges offer advantages with modern machines which exert eccentric force to the seal during crimping, i.e., those that use roller or rail-based technology. A controlled score seal could partially break during such crimping.
FOS Button Designs
Among FOS button designs, Matte-Top is recommended. It is less prone to scratches and scuffing, and better suited to camera inspection (less glare).FOS with a logo embossed can be used in all regions, except for the US market. It is not permitted per USP General Chapter <1> Injections and Implanted Drug Products (Parenterals) - Product Quality Tests. Details on this regulation are discussed in section titled Spectra™ FOS.
Spectra™ FOS
An optional add-on technology for FOS is Spectra™ FOS technology. Spectra™ FOS technology is West’s proprietary method for placing messages on a FOS – to provide point-of-use instructions, product authentication, and counterfeiting deterrence. It comprises printing and/or applying in-mold text on the plastic button and/or printing on the aluminum shell.2
It is noted that all drug products offered in the US must meet the requirements in Chapter <1> regarding labeling. According to Chapter <1>, only cautionary statements intended to prevent imminent life-threatening situations are permitted to appear on the top circle area of the ferrule (i.e., aluminum shell) and the plastic cap (i.e., button). Additionally, the cautionary statements should be printed in a contrasting color and should be clearly visible under ordinary conditions of use.
Seal Grades
West has developed Flip-Off® seals in different product grades to address different fill-finish and regulatory requirements2:
- Standard FOS: for consistent, reliable crimping outside controlled environments
- Flip-Off® Plus (FO Plus) seals: for clean crimping under grade A air supply
- Flip-Off® Clean, Certified, Sterilized (FOCCS) seals: for aseptic crimping inside grade A environments
Standard FOS (except ready-to-use irradiated, RUI) are supplied as assembled without any further processing; they do not come with any cleanliness specification. On the other hand, FO Plus seals and FOCCS seals are certified for pre-sterilization bioburden, to meet the guidelines of the latest European Medicines Agency (EMA) Good Manufacturing Practice (GMP) Annex1 – Manufacture of Sterile Medicinal Products (more guideline details are provided below). This is achieved by measures to control cleanliness in manufacturing, such as specified gowning and sanitization of assembly equipment contact surfaces.
EMA GMP Annex1 Manufacture of Sterile Medicinal Products”3
Some key features are:
- Clause 80: The bioburden should be monitored before sterilization. There should be working limits on contamination immediately before sterilization, which are related to the efficiency of the method to be used. Bioburden assay should be performed on each batch for both aseptically filled product and terminally sterilized products…
- Clause 120: Vial capping can be undertaken as an aseptic process using sterilized caps or as a clean process outside the aseptic core. Where this latter approach is adopted, … stoppered vials should be protected with a Grade A air supply until the cap has been crimped.
This guideline applies to manufacturing in Europe and to companies that want to export to Europe.
For FOCCS seals, manufacturing cleanliness control is further enhanced by processing and surface treatment measures, which include washing of the plastic buttons and applying an additional silicone coating to the aluminum shells prior to assembly with the washed buttons. These allow the FOCCS seals to have further reduced bioburden levels and visible particle levels respectively. For more details about FOCCS seals and FO Plus seals, please see the report by West scientists: TR 2014/158: West Flip-Off® Seals for Aseptic and Clean Crimping Processes.
West neither washes assembled seals, nor recommends washing. This is because there is a risk of the aluminum shells deforming, especially for larger seals. Moreover, the seals may undergo stress by collision or abrasion during the washing process, which may result in formation of particles, so particle levels may be potentially higher after washing.4
The three product grades are offered as gamma-sterilized or ready-for-sterilization:
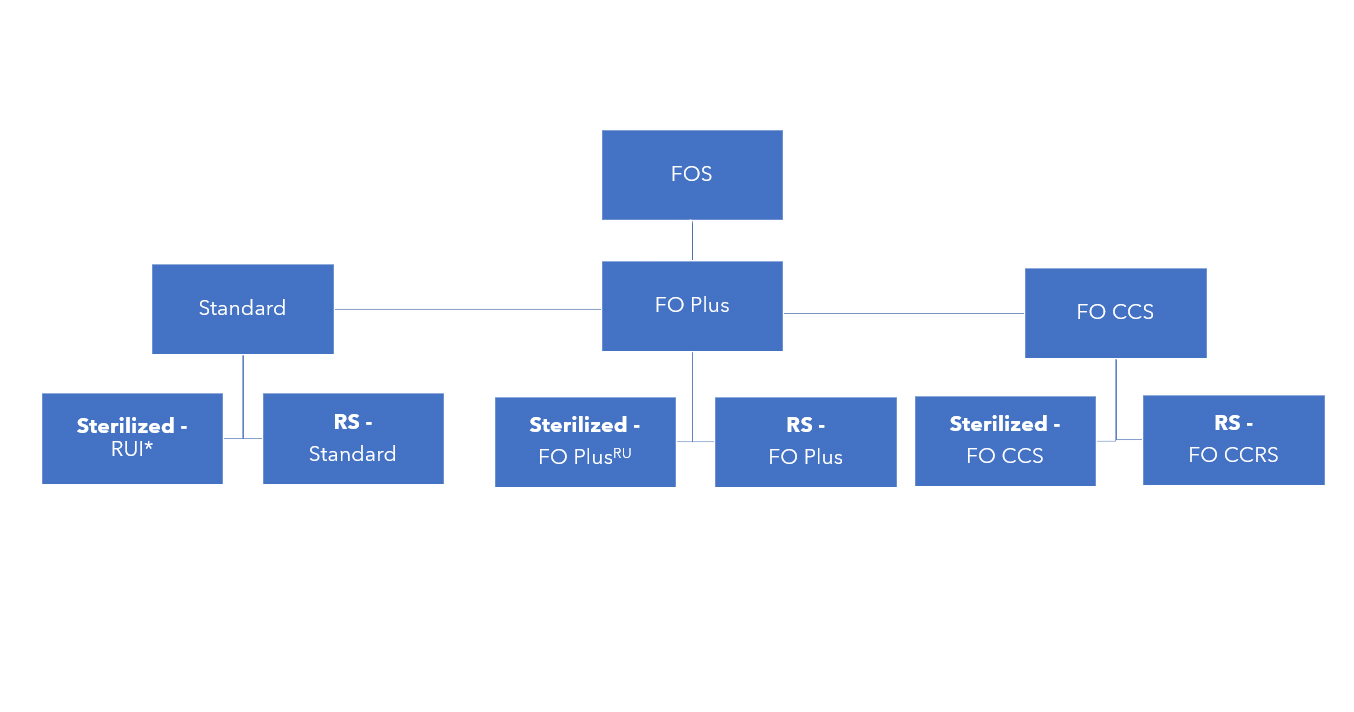
*RUI: ready-to-use irradiated
West performs sterilization by gamma irradiation, instead of steam, to avoid the possibility of excess condensate being trapped under the seal buttons, which would then require further drying.
The plastic buttons used for gamma-irradiated sterilized seals are made of gamma-stable polypropylene (PP) resin, instead of standard PP resin, which may discolor and become brittle following gamma irradiation. If irradiation is required, gamma-stable buttons may be specified.
Follow up reading
The Knowledge Center contains a wealth of information on seals. An easy read is our Flip-Off® Seals Product Technical Overview, and background on colors and color terminology can be found here.For more discussion about seal types and recommendations, please contact the West Technical Customer Support Team or visit the West Knowledge Center.
Note: Lined seals are not discussed within this blog as they are not a secondary component and do come in direct contact with drug products.
Flip-Off, Flip Off Tear Off and Spectra are trademarks and registered trademarks of West Pharmaceutical Services, Inc., in the United States and other jurisdictions.
References:
- Deutsch, M. (2017, October 20). Westpharm.com. Adding Color to Your Closure Choices. https://www.westpharma.com/blog/2017/October/adding-color-to-your-closure-choices
- Boo, JM. (2017, August 03). Westpharma.com. West Flip-Off® Seals – A Diverse Portfolio. https://www.westpharma.com/blog/2017/August/west-flip-off-seals---a-diverse-portfolio
- EUROPEAN COMMISSION; EudraLex; The Rules Governing Medicinal Products in the European Union; Volume 4: EU Guidelines to Good Manufacturing Practice, Medicinal Products for Human and Veterinary Use; Annex 1: Manufacture of Sterile Medicinal Products.
- Boo, JM. (2018, April 25). Westpharm.com. Recommendations on Processing West Flip-Off Seals. https://www.westpharma.com/blog/2018/April/recommendations-on-processing-west-flip-off-seals













