West FluroTec® Prefillable Syringe Plungers: A Solution for Low Temperature Storage
Mitigate risk of contamination, enable accurate dosing, while maintaining the stability and sterility of parenteral products
Selecting the right primary container presentation for a drug application is an important decision that can directly impact patients. Prefillable syringes (PFS) is one of the fastest growing segments within the injectables market. The growth is driven by strong biological pipeline, combination products, home care and self-administration.
There are many reasons why pharmaceutical companies are interested in converting from Multi Dose Vials [MDV] into a safe syringe system. One is to have a more convenient patient-centric administration format. Prefilled syringes are filled with accurate dosing and thus reduce the risk of medication errors in a ready-to-use format. By using prefillable syringes, the risk of microbiological contamination decreases, due to the lower amount of preparation steps and less time needed by healthcare providers to prepare the drug for injection. Further argument for the use of prefilled syringes is that prefilled syringes also have less overfill compared to MDVs, which helps prevent drug waste.
While during the pandemic of the COVID-19 virus, various sizes of MDV formats were approved by the authorities and have proven to be a highly effective containment solution, allowing high vaccination rates (13.5 bn vaccine doses administered worldwide through August 2023*), a transition towards single dose applications such as prefilled syringe container solutions during the endemic phase might be appropriate.The development of mRNA [messenger ribonucleic acid] vaccines set a path for whole new class of promising mRNA-based vaccines for infectious diseases and cancer, mRNA treatment for food and environmental allergies, and a platform for genetic diseases.
One of the challenges with highly sensitive mRNA-based vaccines is that they must be maintained at low temperatures. While data exists for cold storage in MDV, less experience is available for prefilled syringes. The prefilled syringes serve as both the container for storage and the drug delivery device. Therefore, prefilled syringes must be carefully evaluated to high standards regarding performance such as the container closure integrity, break loose and extrusion forces, and plunger movement during cold storage.
With the need for more high value drug products to be stored at low temperatures for shelf life, drug conservation and patient convenience, PFS seems like a logical solution. For room temperature storage, this is perfect. Drugs that need sub-ambient storage come with additional concerns for storage including leakage, syringe cracking, and sterility breaches by plunger movement. These concerns are independent of whether the syringe is a polymer or glass-based system, or the level of siliconization.
Overall, the number of drug products requiring storage in temperatures lower than -20°C has risen significantly in the past few years. There are many reasons for frozen conditions as e.g., retarding degradation, extending shelf life, and reducing leachables attributed to the container closure.
The growing trend of mRNA applications, especially vaccines, in the Advanced Therapeutics Biologics space is moving to prefilled syringes, requiring a plunger that can function at low temperatures down to -50°C.
Select Prefillable Syringe FluroTec® plunger designs meet these criteria and work with ISO glass syringes that are currently available in the market.
There are challenges for the plunger components when looking at low temperature storage. The syringes and plungers were designed to fit and function at room temperature. More specifically, they are designed to maintain sterility, container closure integrity, and enable acceptable break loose and extrusion forces (BLG/BLE) forces during administration. For any PFS intended for use under frozen conditions, it must be determined experimentally whether acceptable performance can be achieved. The characterization of the plunger performance in a prefillable syringe is essential to determine the suitability for intended use at low temperature.
The following sections give insights from observations obtained based on a performance study conducted with glass and plastic prefilled syringes in combination with FluroTec plungers using a well understood bromobutyl rubber formulation, 4023/50 grey, during and after freeze-thaw exposure.
The FluroTec™ barrier film lamination used for these respective plungers, is a widely proven Ethylene tetrafluoroethylene (ETFE) film which acts as a barrier reducing interaction of drug product with the elastomeric plunger and limiting potential plunger leachables into the drug product, thereby reducing the potential of contaminants which may impact drug efficacy and safety. Challenges with storage/delivery of COVID-19 vaccine in a PFS are amplified since vaccine contact time may be longer with a plunger than with a stopper.As pharmaceutical companies seek to move from vials to prefilled syringes for mRNA vaccines, there is a lack of data demonstrating performance and CCI (Container Closure Integrity) at low temperatures, including -50°C. Additionally, there is no standard method for testing CCI on prefilled syringes. All of this is making it difficult for mRNA drug developers to choose and validate proper prefillable syringe components for their drugs.
We conducted a study with two FluroTec plunger designs, the 1 mL Long and the 1-3 mL. The evaluation was conducted with 1 mL long glass and polymer-based syringes, and with 2.25 mL glass syringes, respectively. West has developed methods for testing plunger movement, BLE (Break Loose and Extrusion Force) and CCI for syringes that undergo cold storage processing, and results of the tests are discussed. The BLE method is validated, while the plunger movement and CCI methods were developed by West.
The following tests have been completed:
- Plunger movement during storage at low temperature: The prefilled syringe system might be affected by pressure change, e.g., during air transport or low temperature and freezing/thawing cycles. When a drug product freezes, the liquid expands and, depending on the headspace, that expansion can physically push the plunger distally into the non-sterile area of the barrel. When the syringe is brought back to room temperature, any foreign material could be brought back into the sterile area, hence there is a potential risk of contaminating the drug product. Furthermore, moving the plunger from its original position can potentially alter the initial expected break-loose value of the syringe system.
Experimental evaluation was made of water-filled PFSs comprising ISO-standard 1 mL long and 2.25 mL glass staked-needle syringes with FluroTec® plungers. Storage conditions were at -50°C (passive freezing in mechanical freezer, vis-à-vis controlled-rate freezing) for > 24 hours followed by bench-top thaw for > 1 hour. In this experiment, the plunger position is recorded before freezer placement, immediately after removal, and after thaw. Emphasis is on movement past the sterile barrier as defined by the sealing ribs.
- Container closure integrity (CCI) plays a crucial role in maintaining the stability and sterility of parenteral products throughout their shelf life. Container closure integrity may also be affected by extremely low temperatures such as -80°C due to a loss of elasticity in the rubber components and the varying shrinkage rates of different materials. For example, it is not uncommon to see that the syringe barrel shrinks less than the plunger, which could result in minimal interference and a breach in the barrier against microbial ingress. In this case, CCI was determined by laser-based headspace analysis for oxygen by comparing values as assembled and after freezer placement and thaw. The test was conducted with 1 mL long glass barrels paired with FluroTec long plunger and 2.25 mL glass barrels paired with FluroTec laminated 1-3 mL plunger. Syringes were divided into three groups: RT (Room Temperature), -40°C, and -50°C storage conditions.
- Performance: Break Loose and extrusion Force [BLE]: Break-loose and extrusion forces are measured by determining the force needed (with tensile testing equipment, e.g., by Instron Corp.) to initiate plunger movement and sustain a desired plunger movement rate. Comparison is made of samples before and after freezer placement and thaw. The test was conducted with 1 mL long glass barrels paired with FluroTec laminated 1 mL long plungers and 2.25 mL glass barrels paired with FluroTec laminated 2-3 mL plungers. Syringes were divided into two groups: RT and -50°C storage conditions. The BLE was performed according to ISO 11040-8, section 6.2 with a speed of 100 mm/min.
Results:
Plunger movement triggered by lower temperature largely depends on the fill volume. The data that was generated shows that smaller fill volumes result in less movement. In the study conducted, the 1 mL long syringe filled at 0.5 mL movement never exceeded 1.5 mm, while the syringes filled to 1 mL movement reached a 3.5 mm distance. The sterile barrier zone for the plungers used in this study was estimated based on drawings at room temperature in an uncompressed state, and was determined to be around 4 mm. Therefore, to minimize risk of breaching sterile barrier, not filling syringes to their maximum capacity may be a potential approach. The headspace, material of the barrel, design of the plunger and orientation of the storage used in this study don’t seem to affect plunger movement in a significant way. It should be noted that the headspace influences plunger movement in plungers shipped by air transport and can create an additive effect to the movement observed, which is outside the scope of this report. An example of the results is shown in figure 1for the 1 mL long glass combination with two filling volumes.
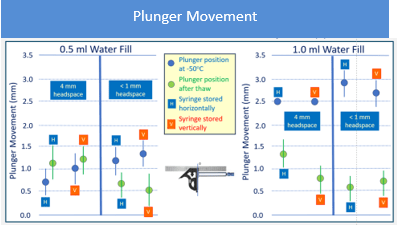
Figure 1: 1 mL FluroTec plunger and 1 mL long glass syringe after storage at -50°C for 24 hours and thaw. The sterile barrier is not passed.
CCI testing showed no gross gas exchange during the -40°C and -50°C freezing. Gross exchange is defined as a 2% or higher increase in oxygen concentration, which was experimentally determined. When stored at cold temperatures, the exchange rates were significantly less, and no values were seen > 2%. These results indicate that the assemblies of the 1 mL long FluroTec plungers in a standard 1 mL long glass syringe and in a standard 1 mL polymer-based syringe, and the 1-3 mL FluroTec plungers in a standard 2.25 mL glass syringe exhibited promising behavior that warrant further study for container closure at low temperatures. The evaluation of CCI demonstrated no breach of any PFS. Example data are shown in Figure 2; the oxygen concentration was measured before, and after, freezer placement and thaw. No increase in oxygen concentration was observed. A breach would result in an oxygen concentration the same as freezer air (i.e., 21%), especially since the decrease in pressure within PFS upon freezer placement would promote air ingress.
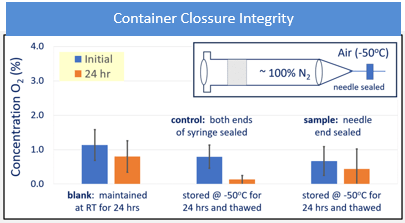
Figure 2: 1 mL FluroTec plunger and 1 mL long glass syringe after storage at -50°C for 24 hours and thaw.
The syringes tested for BLE post -50°C storage showed similar appearances. Overall, the evaluation of initiation and sustaining forces at 100 mm/min demonstrated no change in performance for any PFS resultant from freezer placement and thaw. Syringes with FluroTec® laminated plungers were tested for break-loose and extrusion forces post -50°C storage glass syringes showed typical BLE values and performance at both sizes. The 1 mL polymer syringe showed a slightly higher break-loose, maximum extrusion and average extrusion than the glass syringes, but is still comparable to the glass performance. Example data are shown in figure 3.
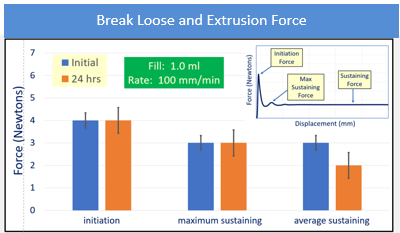
Figure 3: 1mL FluroTec plunger and 1 mL long glass syringe after storage at -50°C for 24 hours and thaw. The sterile barrier is not passed.
It should be noted that all the tests performed here are meant to demonstrate examples of the developed tests and not necessarily real-life situations. As noted in the experimental section, test method elements such as models and materials of the barrels, plungers, and plunger rods, plunger placement method and headspace, fill media and volume, storage temperate and other conditions should be selected based on the intended use. Additionally, other parameters not tested in this work might need investigation, such as air transportation simulations, particle analysis and material properties to determine suitability of the prefilled packaging system for cold storage. Overall, it can be concluded that these testing approaches appear generally suitable for evaluating prefilled syringes systems at low temperatures.
References:
- (*) World Health Organization _Coronavirus disease (Covid -19) Numbers at a glance [status 21 August 2023]
- FROM VIAL TO PREFILLED SYRINGE: MIGRATING A DRUG PRODUCT PRESENTATION - ONdrugDelivery
- Source Conclusion TR 2023/266 page 10
- Citeline UK Ltd 2023 in cooperation with West: Article COVID -19 spurs boom in both market and innovation for injectable vaccines in cooperation with West
- FROM VIAL TO PREFILLED SYRINGE: MIGRATING A DRUG PRODUCT PRESENTATION - ONdrugDelivery
- Transitioning mRNA Covid-19 vaccines from vial to prefilled syringe | SCHOTT Pharma (schott-pharma.com)
- 2023 AAPS PharmSci 360, Poster, Performance of PreFilled Syringe Systems at Low Temperature Louis Brasten, Olga Laskina, Page McAndrew
FluroTec is a trademark of West Pharmaceutical Services, Inc. in the United States and other jurisdictions, and FluroTec technology is licensed from Daikyo Seiko, Ltd.






