An Introduction to Parenteral Packaging: The Backbone of Safe and Effective Injectable Drug Delivery
In an industry defined by innovation, one essential element quietly shapes the success of every injectable therapy: parenteral packaging. Parenteral packaging may sound technical, but its purpose is straightforward - it is the primary container system that holds, protects, and delivers sterile drug products to patients.
“Parenteral” refers to drug products administered by injection, bypassing the gastrointestinal tract. Depending on therapeutic requirements, these injections are delivered through several main routes, each chosen to optimize onset, duration, and patient experience (see illustration and table below).
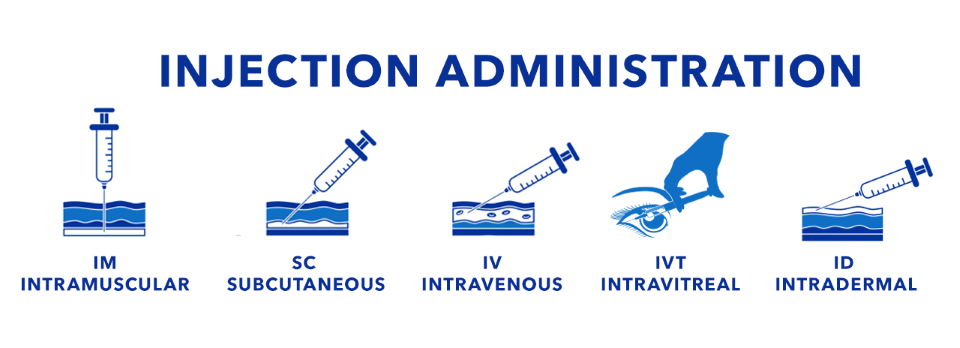
Routes of Injection – Summary Table| Route | Description | Target Site | Onset of Action | Common Uses |
|---|
| Intravenous (IV) | Drug is injected directly into the bloodstream via a vein | Vein (systemic circulation) | Immediate (seconds to minutes) | Emergency medications, chemotherapy, IV fluids, anesthetics |
| Intramuscular (IM) | Drug is injected into a muscle, allowing gradual absorption into circulation | Skeletal muscle (e.g., deltoid, gluteus, thigh) | Moderate (5–30 minutes) | Vaccines, antibiotics, hormonal therapies, depot injections |
| Subcutaneous (SC) | Drug is injected into fatty tissue beneath the skin for slower absorption | Subcutaneous tissue (fat layer under skin) | Slow (30–60 minutes) | Insulin, heparin, monoclonal antibodies, GLP-1 agonists |
| Intradermal (ID) | Drug is delivered into the dermis, just below the epidermis skin layer | Dermal layer of skin | Very slow / localized | Allergy testing, TB (Mantoux) test, vaccine research |
| Intravitreal (IVT) | Drug is injected into the vitreous humor of the eye for retinal delivery | Posterior segment of the eye (vitreous cavity) | Localized (retinal level, prolonged effect) | Macular degeneration, diabetic retinopathy, retinal vein occlusion |
From vials and pre-filled syringes to cartridges and stoppers, each component of the parenteral packaging system must meet stringent standards to ensure sterility, compatibility, and consistent performance throughout a drug product’s lifecycle.
Because the packaging is in direct contact with the drug product, its container closure components play a pivotal role in maintaining drug quality and safety. They preserve stability, maintain closure integrity, ensure usability, and protect against contamination to instill confidence from development through delivery.
Pharmaceutical packaging can be envisioned as a two-layer system that safeguards the drug product and patient. The primary packaging forms the innermost layer and consists of components that directly contact the drug product. This includes components such as stoppers, vials, cartridges, and pre-fillable syringes. Encasing the primary layer is the secondary packaging which comprises cartons, trays, and labeling. Serving as the outer shield, secondary packaging protects the primary container during handling and transport, enables clear identification, and supports efficient distribution across the supply chain.
Together, these packaging layers work in harmony to do more than simply contain a product – they protect the integrity of life changing therapies. Ultimately, parenteral packaging serves as a critical partner in enabling safe, effective, and reliable therapies for patients around the world.
Parenteral Packaging and Critical Components
Parenteral packaging is often categorized by format, and common types include vials, pre-filled syringes (PFS), and cartridges. However, the performance of these systems relies just as much on the components paired with them, such as elastomeric stoppers or plungers and aluminum crimp caps. These elements play a vital role in ensuring functionality, maintaining container closure integrity, and preserving sterility throughout a drug product’s lifecycle.
Primary Packaging Types| Common Types | Used For | Made Of | Why Choose |
|---|
| Vials | Multi-dose applications,
lyophilized (freeze-dried) drug products,
broad therapy range | Glass or polymer vial
Requires closure with an elastomeric stopper, seal, press-fit closure, aluminum crimp cap | Versatile, stable, widely accepted;
easy to scale for commercialization |
| Pre-filled Syringes (PFS) | Ready-to-administer biologics, vaccines | Staked needle or luer lock glass or polymer barrel
Requires an elastomeric plunger (with cavity), plunger rod, needle shield (soft or rigid) | Reduces preparatory steps,
improves dosing accuracy |
| Cartridges | Pens, wearable injectors,
multi-dose self-administration devices | Glass or polymer barrel
Requires an elastomeric plunger (without cavity), aluminum cap with elastomeric septum | Supports adherence,
intuitive for chronic use,
integrates with devices |
Each packaging format meets different clinical, manufacturing, and user needs, and all share a common purpose: maintaining sterility and stability throughout a drug products lifecycle, from production through patient administration.
Selecting Packaging Early Supports Success
Parenteral packaging decisions directly influence nearly every stage of drug development. From drug formulation compatibility and sterile fill finish operations to device design, stability studies, and regulatory submissions. Making an informed decision early can help reduce costly delays and support a smoother path to commercialization.
Selecting a well characterized, high quality packaging system early in development is key to ensuring drug performance, patient safety, and time to market success.
Partnering for Packaging Excellence
At West, we understand that parenteral packaging is a critical decision to protect, store, and deliver precious drug products. Our Technical Customer Support (TCS) team partners with pharmaceutical and biotech developers at every stage of drug development to identify the best packaging options depending on the specifics including molecule and delivery. We would love to partner with you. Get in touch here to start the conversation.
Stay Tuned
Over the next two installments, we will take a closer look at how to select the right packaging system and explore the materials and technical considerations that shape performance across development.



