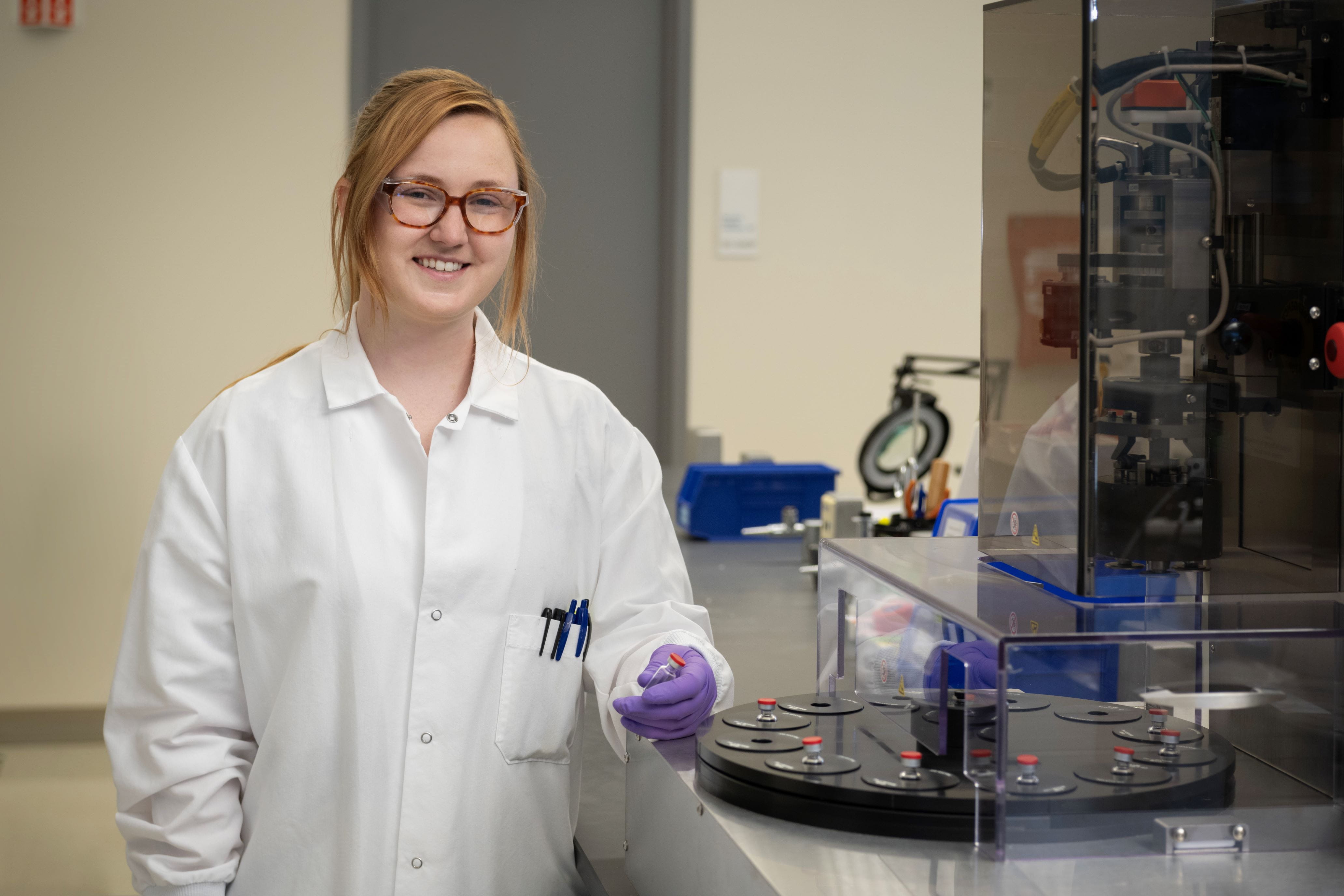
West offers a comprehensive range of Container Closure Integrity (CCI) technologies for diverse packaging and delivery systems, supporting pharmaceutical, biotech, and medical device manufacturers. By partnering with West's CCI experts, companies benefit from exceptional customer support and technical expertise, enabling flexibility and innovation in packaging solutions. This collaboration can enhance drug product value and ensure patient safety.
West's CCI technologies are tailored to package type, product fill, and storage conditions. Our analysts evaluate parenteral packaging systems using probabilistic and deterministic methods, aligning with USP <1207> guidelines. They assist customers from package development to life cycle management, navigating regulatory landscapes.
With over 20 years of experience, West's analytical services team provides method development and validation tailored to your product. They offer customized CCI studies for specific product lifecycle phases